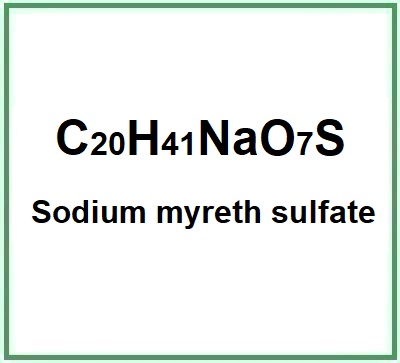
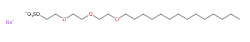
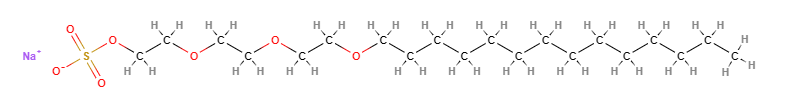
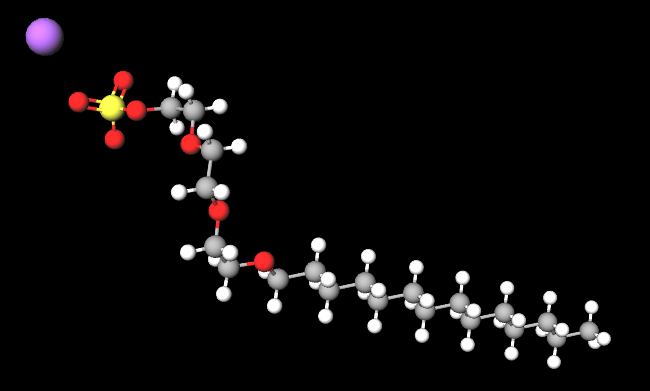
Sodium myreth sulfate is a chemical compound, an anionic surfactant, ethoxylated (reaction by polymerisation of ethylene oxide).
The name defines the structure of the molecule:
- Sodium (Na). Sodium is an alkaline metal, a chemical element with the symbol Na (from the Latin "Natrium") and the atomic number 11. Sodium is soft and silvery-white in color. It is highly reactive, especially with water, and is commonly found in salts and minerals.
- Myreth refers to a synthetic compound that is derived from miristyl alcohol, a fatty alcohol that is often used in cosmetics and personal care products.
- Sulfate refers to a salt or ester of sulfuric acid. Sulfates are often used in cosmetics and personal care products for their ability to create a foam, common in shampoos and body washes.
The synthesis process takes place in several stages:
- Ethoxylation. Myristyl alcohol, which is obtained from natural sources such as coconut oil or can be synthesized from myristic acid and made to react with ethylene oxide in a treatment called ethoxylation. The number of ethylene oxide units added varies in general, but for sodium myrtle sulfate, the "myreth" indicates that on average 10 units of ethylene oxide per molecule of Myristyl alcohol are added.
- Sulfation. Myristyl alcoholl ethoxylate is reacted with sulfur trioxide (SO3) to add a sulfate group, forming the corresponding sulfuric acid ester.
- Neutralization. The sulfuric acid ester is neutralized with sodium hydroxide (NaOH) to form Sodium Myreth Sulfate.
It typically appears as a clear to yellowish liquid at room temperature. It is water-soluble and is often used in aqueous solutions.

What it is used for and where
Cosmetics
Surfactant-foaming (1) emulsifying agent that facilitates amalgamation between the various ingredients. Emulsifiers have the property of directly influencing the stability, sensory properties and surface tension of sunscreens by modulating their filmometric performance.
INCI functions:
Cleansing agent. Ingredient that cleanses skin without exploiting the surface-active properties that produce a lowering of the surface tension of the stratum corneum.
Foaming. Its function is to introduce gas bubbles into the water for a purely aesthetic factor, which does not affect the cleaning process, but only satisfies the commercial aspect of the detergent by helping to spread the detergent. This helps in the commercial success of a cleansing formulation. Since sebum has an inhibiting action on the bubble, more foam is produced in the second shampoo. In practice, it creates many small bubbles of air or other gases within a small volume of liquid, changing the surface tension of the liquid.
Surfactant - Cleansing agent. Cosmetic products used to cleanse the skin utilise the surface-active action that produces a lowering of the surface tension of the stratum corneum, facilitating the removal of dirt and impurities.
Surfactant - Emulsifying agent. Emulsions are thermodynamically unstable and are used to soothe or soften the skin and emulsify, so they need a specific, stabilising ingredient. This ingredient forms a film, lowers the surface tension and makes two immiscible liquids miscible. A very important factor affecting the stability of the emulsion is the amount of the emulsifying agent. Emulsifiers have the property of reducing the oil/water or water/oil interfacial tension, improving the stability of the emulsion and also directly influencing the stability, sensory properties and surface tension of sunscreens by modulating the filmometric performance.
Use
- Shampoo 1%
- Shower 8%
- Hair dye 2,5%
Safety
After the ethoxylation procedure, scientific literature reports that it is not uncommon to find residues of ethylene oxide and 1,4-dioxane, chemical compounds that are considered carcinogenic, in the final product. It would therefore be necessary for the manufacturer to declare the purity of the ethoxylated chemical to be sure that the ingredient is harmless.
The CIR Expert Panel is of the opinion that Sodium myreth sulfate is safe as a cosmetic ingredient in the concentrations and uses established by cosmetic standards (2).
The most relevant studies on this ingredient have been selected with a summary of their contents:
Sodium myreth sulfate studies
| Appearance | Powder |
| PSA | 102.50000 |
| LogP | 5.29490 |
- Molecular Formula C20H41NaO7S
- Molecular Weight 448.6
- Exact Mass 448.24700
- CAS 25446-80-4
- UNII 2VLC033A4E
- EC Number 246-986-8
- DSSTox Substance ID DTXSID6067096
- IUPAC sodium;2-[2-(2-tetradecoxyethoxy)ethoxy]ethyl sulfate
- InChI=1S/C20H42O7S.Na/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-24-15-16-25-17-18-26-19-20-27-28(21,22)23;/h2-20H2,1H3,(H,21,22,23);/q;+1/p-1
- InChl Key MDSQKJDNWUMBQQ-UHFFFAOYSA-M
- SMILES CCCCCCCCCCCCCCOCCOCCOCCOS(=O)(=O)[O-].[Na+]
- MDL number
- PubChem Substance ID
- RTECS KM2931050
Synonyms:
- sodium 2-[2-[2-(tetradecyloxy)ethoxy]ethoxy]ethyl sulphate
- Polyethylene glycol (1-4) myristyl ether sulfate,sodium salt
- Ethanol,2-(2-(2-(tetradecyloxy)ethoxy)ethoxy)-,hydrogen sulfate,sodium salt
- 2-(2-(2-(Tetradecyloxy)ethoxy)ethoxy)ethanol hydrogen sulfate sodium salt
References_____________________________________________________________________
(1) Petrovska, L. S., Baranova, I. I., Bezpala, Y. O., & Kovalenko, S. M. (2017). The study of physicocochemical parameters of some detergents with the anionic nature.
Abstract. The physico-chemical parameters of etergents with the anionic nature, namely sodium myreth sulfate and sodium lauryl sarcosinate used most often when developing domestic foaming products for personal hygiene, at the cosmetic enterprises of Ukraine have been studied and analyzed. The indica-tors of the foaming ability (foam number, foam stability) of the test samples at different pH values, namely at the neutral pH (5.5 -6.0) and at the acid рН (3.5-4.0), have been determined and studied. The study of the foam structure at the pH intervals under research has been also conducted using the method of microphotography. According to the results of the study, the conclusion has been made that different pH values do not affect the foaming ability; however, they affect the foam structure.
(2) Final Report on the Safety Assessment of Sodium Myreth Sulfate. Journal of the American College of Toxicology. 1992;11(1):157-163. doi:10.3109/10915819209141996
Abstract. Sodium Myreth Sulfate is the sodium salt of sulfated, ethoxylated myristyl alcohol which is used as a surfactant and cleansing agent in cosmetics at concentrations ranging from > 1.0–5.0% to > 50.0%. A formulation containing 7.0% Sodium Myreth Sulfate was shown to be an ocular irritant in experimental animals and in some human test subjects. These irritant effects were similar to those previously reported for the chemically similar compound Sodium Laureth Sulfate which was shown to be safe for use in cosmetics. The report summarizes the safety test data on Sodium Laureth Sulfate. Based upon the combined data cited in the report on both cosmetic ingredients, it is concluded that Sodium Myreth Sulfate is safe as a cosmetic ingredient in the present practices of use and concentration.
![]() Sodium myreth sulfate
Sodium myreth sulfate