Acetamide MEA (Acetamine monoethanolamine) is a chemical compound, an aliphatic amide of acetic acid belonging to the class of ethanolamines, a chemical family of surfactants and emulsifiers.
The name defines the structure of the molecule
- "Acetamide". It's an organic compound derived from acetic acid. It has the chemical formula CH3CONH2 and acts as the amide of acetic acid.
- "MEA" stands for "Monoethanolamine". It's an organic compound used as an alkalinizing agent and emulsifier.
Description of the raw materials used in its production:
- Acetamide - An organic molecule derived from acetic acid.
- Monoethanolamine (MEA) - A primary amine and alcohol which has various applications in the chemical industry.
Industrial chemical synthesis of Acetamide MEA, step by step:
- Reaction - Acetamide is reacted with monoethanolamine in a controlled environment. During this reaction, the monoethanolamine acts as a base, neutralizing the acetamide and forming the salt, known as Acetamide MEA.
- Isolation - After the reaction reaches completion, the mixture is cooled to allow for precipitation of the formed salt.
- Filtration - The solution is then filtered to separate the solid Acetamide MEA from the remaining liquids.
- Drying - The solid Acetamide MEA is then dried to remove any remaining moisture.
- Quality control - Once Acetamide MEA is obtained, it undergoes various quality checks to ensure it meets specifications.
It appears as a fine white powder, very stable with good hydroscopicity.

What it is used for and where
Cosmetics
Conditioning-humectant and hair conditioning agent, stabiliser. Proven to reduce eye irritation (1) and is a good dispersing agent for dyes/pigments allowing easy incorporation of these materials into make-up with uniform deposition on application.
It acts in this way:
Hair conditioning agent. A large number of ingredients with specific purposes can co-exist in a hair shampoo: cleansers, conditioners, thickeners, mattifying agents, sequestering agents, fragrances, preservatives, special additives. However, the indispensable ingredients are the cleansers and conditioners as they are necessary and sufficient for hair cleansing and manageability. The others act as commercial and non-essential auxiliaries such as: appearance, fragrance, colouring, etc. Hair conditioning agents have the task of increasing shine, manageability and volume, and reducing static electricity, especially after treatments such as colouring, ironing, waving, drying and brushing. They are, in practice, dispersing agents that may contain cationic surfactants, thickeners, emollients, polymers. The typology of hair conditioners includes: intensive conditioners, instant conditioners, thickening conditioners, drying conditioners.
Skin conditioning agent - Humectant. Humectants are hygroscopic substances used to minimise water loss in the skin and to prevent it from drying out by facilitating faster and greater absorption of water into the stratum corneum of the epidermis. The epidermis is the most superficial of the three layers that make up the human skin (epidermis, dermis and hypodermis) and is the layer that maintains hydration in all three layers. In turn, the epidermis is composed of five layers: corneum, the most superficial, lucidum, granulosum, spinosum and basale. Humectants have the ability to retain in the stratum corneum the water they attract from the air and have the function of moisturising the skin. It is better to use them before emollients that are oil-based.
Surfactant - Foam booster. Their function is to introduce gas bubbles into the water for a purely aesthetic factor, which does not affect the cleaning process, but only satisfies the commercial aspect of the detergent by helping to spread the detergent on the hair. This helps in the commercial success of a shampoo formulation. Since sebum has an inhibiting action on the bubble, more foam is produced in the second shampoo.
Viscosity Enhancing Agent - aqueous. Since viscosity is important for increasing the chemical and physical stability of the product, Viscosity Enhancing Agent acqueous is an important dosage factor in gels, suspensions, emulsions, solutions. Increasing viscosity makes formulations less sedimentary and more homogeneously thickened.
Use: max 7,5%
Safety
Based on the data presented in this rather old EPA study, 1993, it is concluded that Acetamide MEA is safe as a cosmetic ingredient at concentrations not exceeding 7.5 % and is safe in current use in rinse-off products. Cosmetic formulations containing Acetamide MEA should not contain nitrous agents or significant amounts of free acetamide (2). In subsequent years, it has been reconfirmed that Acetamide MEA at concentrations not exceeding 7.5% is considered safe for human health by the Annual Review of Cosmetic Ingredient Safety Assessments in 2011 (3).
Commercial applications
Food. It's used as a preservative and stabilizer. It also acts as an acidity regulator in drinks and foods, such as ice creams and jellies.
Pharmaceuticals. Used as an alkalinizing agent in dialysis solutions and as an anticoagulant in blood for transfusions.
Water Treatment. Used as a softening agent in water softeners.
Chemical Industry. Used in the production of detergents and soaps to adjust pH.
Acetamide MEA studies
| Appearance | White powder |
| Boiling Point | 151-155 °C5 mm Hg(lit.) |
| Melting Point | 15.8°C |
| Flash Point | 176,66°C |
| Density | 1.12 g/mL at 25°C(lit.) |
| Vapor Pressure | 0.00021mmHg at 25°C |
| Index of Refraction | Index of Refraction |
| PSA | 49.33000 |
| Safety |  |
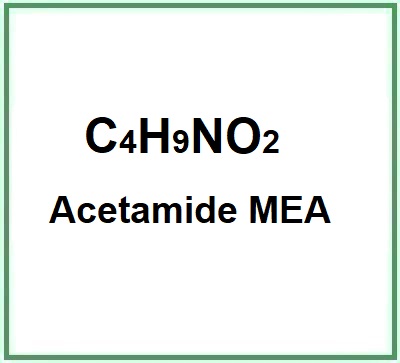
- Molecular Formula C4H9NO2
- Linear Formula CH3CONHCH2CH2OH
- Molecular Weight 103.12
- Exact Mass 103.06300
- CAS 142-26-7
- UNII A9O0818TWD
- EC Number 205-530-8
- DSSTox Substance ID DTXSID6044804
- IUPAC N-(2-hydroxyethyl)acetamide
- InChI=1S/C4H9NO2/c1-4(7)5-2-3-6/h6H,2-3H2,1H3,(H,5,7)
- InChl Key PVCJKHHOXFKFRP-UHFFFAOYSA-N
- SMILES CC(=O)NCCO
- MDL number MFCD00002836
- PubChem Substance ID 24846527
- ChEBI 74687
- RXCUI 1311639
- NSC 5999
- RTECS AC3120000
- NACRES NA.22
Synonyms
- N-(2-hydroxyethyl)acetamide
- 2-Acetamidoethanol
- N-Acetylethanolamine
- N-Acetyl ethanolamine
- Hydroxyethyl acetamide
- N-2-Hydroxyethylacetamide
- N-Acetyl-2-aminoethanol
References___________________________________________________________________
(1) Shercomid AME-70 Technical Bulletin.Clifton.NJ:Scher CDhemicals July 1977
(2) Johnson, W. (1993). Final report on the safety assessment of acetamide MEA. J Am Coll Toxicol, 12, 225-236.
(3) Andersen, F. A. (2011). Annual review of cosmetic ingredient safety assessments: 2007-2010. International Journal of Toxicology, 30(5_suppl), 73S-127S.
![]() Acetamide MEA
Acetamide MEA