![]() Dimethylsilanediol
Dimethylsilanediol
Rating : 6
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
10 pts from CarPas
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Dimetichonol studies" about Dimethylsilanediol Review Consensus 10 by CarPas (5242 pt) | 2022-May-01 12:43 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Xu S. Extraction and quantitative analysis of water by GC/MS for trace-level dimethylsilanediol (DMSD). J Chromatogr A. 2019 Aug 30;1600:1-8. doi: 10.1016/j.chroma.2019.04.026.
Abstract. Dimethylsilanediol (DMSD) is related to the most important bifunctional building block for silicone oligomers and polymers, although DMSD itself is not used in any commercial applications.
Lin Y, Wang L, Krumpfer JW, Watkins JJ, McCarthy TJ. Hydrophobization of inorganic oxide surfaces using dimethylsilanediol. Langmuir. 2013 Feb 5;29(5):1329-32. doi: 10.1021/la303963q.
Abstract. We report that dimethylsilanediol is a useful reagent for the surface modification (hydrophobization) of oxidized silicon and other oxidized metal surfaces and compare the wetting properties of modified solids with those of conventionally modified surfaces.
Xu S, Kropscott B. Method for simultaneous determination of partition coefficients for cyclic volatile methylsiloxanes and dimethylsilanediol. Anal Chem. 2012 Feb 21;84(4):1948-55. doi: 10.1021/ac202953t.
Abstract. Cyclic volatile methyl siloxanes (cVMS) such as octamethylcyclotetrasiloxane (D4), decamethylcyclopentasiloxane (D5), and dodecamethylcyclohexasiloxane (D6) may enter the environment through industrial activities and the use of various consumer products. Reliable air/water (K(AW)), 1-octanol/water (K(OW)), and octanol/air partition coefficients (K(OA)) for those compounds and their common degradation product, dimethylsilanediol, are critical for accurate prediction of the environmental fate, distribution, and transport of these materials. Challenges have been encountered in determining these properties for cVMS and their degradation products mainly due to the extremely low water solubility of the organosiloxanes, low volatility of their degradation products, and reactivity of those compounds in the water/1-octanol system that can lead to inconsistent and inaccurate partition coefficients. A novel direct method is presented for the simultaneous determination of K(AW), K(OW), and K(OA) of organic compounds and was applied to these organosilicon compounds. It was tested in a range of log K(AW) values from -6.8 to 3.1, log K(OW) values from -0.4 to 8.9, and log K(OA) values up to 7.
Duivenvoorden WC, Middleton A, Kinrade SD. Divergent effects of orthosilicic acid and dimethylsilanediol on cell survival and adhesion in human osteoblast-like cells. J Trace Elem Med Biol. 2008;22(3):215-23. doi: 10.1016/j.jtemb.2008.02.001.
Abstract. Although dietary silicon (Si) is recognized to be an important factor for the growth and development of bone and connective tissue, its biochemical role has yet to be identified. The predominant Si-containing species in blood and other biofluids is orthosilicic acid, Si(OH)(4). Dimethylsilanediol, (CH(3))(2)Si(OH)(2), is an environmental contaminant that results from decomposition of silicone compounds used in personal hygiene, health care and industrial products. We examined the in vitro effects of both Si species on the survival (colony forming efficiency), proliferation (DNA content), differentiation (alkaline phosphatase activity) and adhesion (relative protein content) of the human osteoblast-like cell lines Saos-2 and hFOB 1.19.
Lehmann RG, Miller JR, Kozerski GE. Degradation of silicone polymer in a field soil under natural conditions. Chemosphere. 2000 Sep;41(5):743-9. doi: 10.1016/s0045-6535(99)00430-0.
Abstract. Silicone polymers (PDMS = polydimethylsiloxane) are used in numerous consumer and industrial products. Our previous work showed that they will degrade in soil under laboratory conditions. This paper investigates PDMS degradation in the field.
Santos-Clotas E, Cabrera-Codony A, Boada E, Gich F, Muñoz R, Martín MJ. Efficient removal of siloxanes and volatile organic compounds from sewage biogas by an anoxic biotrickling filter supplemented with activated carbon. Bioresour Technol. 2019 Dec;294:122136. doi: 10.1016/j.biortech.2019.122136.
Abstract. The removal of siloxanes (D4 and D5) and volatile organic contaminants (hexane, toluene and limonene) typically found in sewage biogas was investigated in a lab-scale biotrickling filter (BTF) packed with lava rock under anoxic conditions.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Dimethylsilanediol Review Consensus 10 by CarPas (5242 pt) | 2024-Oct-08 17:22 |
| Read the full Tiiip | (Send your comment) |
Dimethylsilanediol is a chemical compound, diol monomer, monomeric unit of polydimethylsiloxane, consisting of 99.9 % dimethylsilanediol and oligomers (1).
Dimethylsilanediol is an organosilicon compound widely used in the cosmetic industry for its moisturizing, regenerative, and antioxidant properties. It belongs to the silane family and is primarily used as a conditioning and protective agent in skincare and haircare products. Due to its ability to enhance skin elasticity and strengthen hair, it is a valuable ingredient in many personal care formulations.
Chemical Composition and Structure
Dimethylsilanediol has a silicon-based chemical structure with two methyl groups attached to a silicon atom and two hydroxyl groups (OH). This structure enables the compound to bind with water, improving hydration and offering antioxidant properties. Silicon, a key component of Dimethylsilanediol, is known for its role in maintaining the integrity of connective tissues, supporting skin regeneration, and strengthening hair.
Physical Properties
Dimethylsilanediol is typically a clear, colorless liquid that is water-soluble and easily incorporated into skincare and haircare formulations. Its film-forming and conditioning properties make it ideal for enhancing the texture and protection of both skin and hair.
Production Process
Dimethylsilanediol is synthesized through chemical reactions involving organosilicon compounds. This process results in a highly pure compound suitable for use in cosmetic and personal care products. It is then stabilized to ensure long shelf life and optimal performance in formulations.
Applications
Skincare: Dimethylsilanediol is used in creams, serums, and lotions to improve hydration and provide antioxidant benefits. It helps strengthen the skin barrier, improve elasticity, and reduce visible signs of aging.
Haircare: In shampoos, conditioners, and hair masks, Dimethylsilanediol strengthens the hair fiber, preventing breakage and enhancing shine and softness.
Cosmetic Products: Used as a conditioning and protective agent, it improves the texture of various formulations, ensuring smooth application and a silky feel.
Health and Safety Considerations
Safety in Use
Dimethylsilanediol is considered safe for use in cosmetic products. It is generally well-tolerated by both skin and hair, and it is not known to cause irritation or sensitization. Its moisturizing and protective properties make it suitable for a wide range of skin types, including sensitive skin.
Allergic Reactions
Allergic reactions to Dimethylsilanediol are rare. However, as with any cosmetic ingredient, a patch test is recommended before using new products, especially for individuals with particularly sensitive skin.
Toxicity and Carcinogenicity
There is no evidence that Dimethylsilanediol is toxic or carcinogenic. It is widely used in cosmetic products and is considered safe at recommended concentrations. Safety studies have confirmed that it does not pose significant health risks to humans.
Environmental and Safety Considerations
Dimethylsilanediol is a biodegradable compound and does not pose significant environmental risks. As part of the silane family, it degrades easily under natural environmental conditions, minimizing its ecological impact.
Regulatory Status
Dimethylsilanediol is approved for use in cosmetics by regulatory bodies such as the European Union and the Food and Drug Administration (FDA) in the United States. It is considered safe for use in a wide range of skincare and haircare formulations.
Safety
Despite the concerns of some in the press about soil pollution by Dimethylsilanediol, the scientific literature has downgraded the problem by showing that degradation is slow and under field conditions as predicted by laboratory experiments (2). Recently found in the ISS space station in a wet zone.
For more information:
Typical commercial product characteristics Dimethylsilanediol
| Appearance | White to Off-white Solid |
| Boiling Point | 122.2ºC at 760mmHg |
| Flash Point | 27.7°C |
| Density | 0.998g/cm3 |
| Loss on drying | ≤2.0% |
| Sulphated ash | ≤0.5%/g |
| Water | ≤1.0% |
| Heavy metals | Heavy metals |
| Impurities | ≤0.5% |
| Purity | ≥99.0% |
| PSA | 40.46000 |
| Vapor Pressure | 6.73mmHg at 25°C |
| Refraction Index | 1.414 |
| Storage | -86°C, |
| Safety |  |
 |  |
 |  |
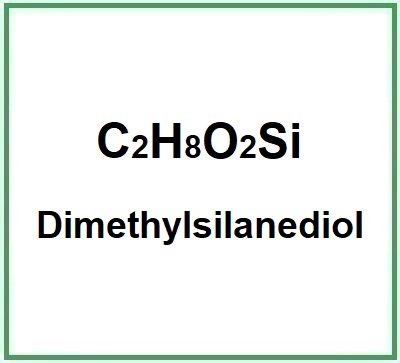
- Molecular Formula C2H8O2Si
- Molecular Weight 92.17
- Exact Mass 92.02940
- CAS 1066-42-8
- UNII
- EC Number 213-915-7
- DSSTox Substance ID DTXSID001019098
- IUPAC dihydroxy(dimethyl)silane
- InChI=1S/C2H8O2Si/c1-5(2,3)4/h3-4H,1-2H3
- InChl Key XCLIHDJZGPCUBT-UHFFFAOYSA-N
- SMILES C[Si](C)(O)O
- MDL number MFCD19441445
- PubChem Substance ID
- ChEBI 23816
Synonyms
- Silanediol, dimethyl-
- Dihydroxydimethylsilane
- dihydroxy(dimethyl)silane
References_________________________________________________________________________
(1) Sabourin CL, Carpenter JC, Leib TK, Spivack JL. Biodegradation of dimethylsilanediol in soils. Appl Environ Microbiol. 1996 Dec;62(12):4352-60. doi: 10.1128/aem.62.12.4352-4360.1996.
Abstract. The biodegradation potential of [14C]dimethylsilanediol, the monomer unit of polydimethylsiloxane, in soils was investigated. Dimethylsilanediol was found to be biodegraded in all of the tested soils, as monitored by the production of 14CO2. When 2-propanol was added to the soil as a carbon source in addition to [14C]dimethylsilanediol, the production of 14CO2 increased. A method for the selection of primary substrates that support cometabolic degradation of a target compound was developed. By this method, the activity observed in the soils was successfully transferred to liquid culture. A fungus, Fusarium oxysporum Schlechtendahl, and a bacterium, an Arthrobacter species, were isolated from two different soils, and both microorganisms were able to cometabolize [14C]dimethylsilanediol to 14CO2 in liquid culture. In addition, the Arthrobacter sp. that was isolated grew on dimethylsulfone, and we believe that this is the first reported instance of a microorganism using dimethylsulfone as its primary carbon source. Previous evidence has shown that polydimethylsiloxane is hydrolyzed in soil to the monomer, dimethylsilanediol. Now, biodegradation of dimethylsilanediol in soil has been demonstrated.
(2) Lehmann RG, Miller JR, Kozerski GE. Degradation of silicone polymer in a field soil under natural conditions. Chemosphere. 2000 Sep;41(5):743-9. doi: 10.1016/s0045-6535(99)00430-0.
Abstract. Silicone polymers (PDMS = polydimethylsiloxane) are used in numerous consumer and industrial products. Our previous work showed that they will degrade in soil under laboratory conditions. This paper investigates PDMS degradation in the field. Four soil plots (each 2.44 m x 2.44 m) in Michigan were sprayed in May, 1997, with aqueous emulsion to achieve nominal soil PDMS concentrations of 0 (control), 215 (low), 430 (medium), and 860 (high) microg/g. Over the following summer, soil cores (0-5 and 5-10 cm) were collected every two weeks and analyzed for decrease in-total soil PDMS, and decrease in molecular weight of remaining PDMS. PDMS concentrations decreased 50% in 4.5, 5.3, and 9.6 weeks for the low, medium, and high treatments, respectively. Degradation rates were 0.26 (low), 0.44 (medium), and 0.44 (high) g PDMS/m2 day, indicating that degradation capacity of the soil was exceeded by the High treatment. Dimethylsilanediol (DMSD), the main degradation product, was detected in most samples at <5% of original PDMS. This is consistent with laboratory data showing biodegradation and volatilization of DMSD. Deeper sampling (to 20 cm) found only trace amounts of DMSD, and minor downward movement of the polymer. Respraying and subsequent analysis of one plot with a medium treatment in late August showed slow PDMS degradation during the cool, wet fall, followed by a 40% decrease over winter and extensive degradation during the summer of 1998. The study thus shows that PDMS will degrade under field conditions as predicted from laboratory experiments.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2022-05-01 15:37:07 | Chemical Risk: |