Nimesulide
Pros:
Anti-inflammatory (1)Cons:
Take only under medical supervision (1)10 pts from CarPas
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Nimesulide studies" about Nimesulide Review Consensus 10 by CarPas (5242 pt) | 2022-May-03 16:54 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Senna GE, Passalacqua G, Andri G, Dama AR, Albano M, Fregonese L, Andri L. Nimesulide in the treatment of patients intolerant of aspirin and other NSAIDs. Drug Saf. 1996 Feb;14(2):94-103. doi: 10.2165/00002018-199614020-00004.
Abstract. Aspirin (acetylsalicylic acid) and other NSAIDs are responsible for many adverse effects. Among them, pseudo-allergic reactions (urticaria/angioedema, asthma, anaphylaxis) affect up to 9% of the population and up to 30% of asthmatic patients. The mechanisms provoking these reactions have not been fully elucidated, but it appears that inhibition of cyclo-oxygenase (COX) plays a central role. The anti-inflammatory action of nimesulide differs from that of other NSAIDs, possibly because of its chemical structure. In particular, nimesulide is selective for COX-2 and displays additional properties in terms of its effects on inflammatory mediator synthesis and release. For these reasons, nimesulide is generally well tolerated by NSAID-intolerant patients and patients with NSAID-induced asthma. The good tolerability of nimesulide as an alternative drug for use in patients with NSAID intolerance has been demonstrated in a large number of clinical studies.
Wei W, Evseenko VI, Khvostov MV, Borisov SA, Tolstikova TG, Polyakov NE, Dushkin AV, Xu W, Min L, Su W. Solubility, Permeability, Anti-Inflammatory Action and In Vivo Pharmacokinetic Properties of Several Mechanochemically Obtained Pharmaceutical Solid Dispersions of Nimesulide. Molecules. 2021 Mar 10;26(6):1513. doi: 10.3390/molecules26061513.
Abstract. The objective of the present study was to investigate the possibility of improving the solubility and the bioavailability of Nimesulide via complexation with polysaccharide arabinogalactan (AG), disodium salt of glycyrrhizic acid (Na2GA), hydroxypropyl-β-cyclodextrin (HP-β-CD) and MgCO3.
Ferreira RG, Narvaez LEM, Espíndola KMM, Rosario ACRS, Lima WGN, Monteiro MC. Can Nimesulide Nanoparticles Be a Therapeutic Strategy for the Inhibition of the KRAS/PTEN Signaling Pathway in Pancreatic Cancer? Front Oncol. 2021 Jul 20;11:594917. doi: 10.3389/fonc.2021.594917.
Abstract. ....However, there is a need to improve nimesulide through its encapsulation by solid lipid nanoparticles to overcome problems related to the hepatotoxicity and bioavailability of the drug.
Bernareggi A. Clinical pharmacokinetics of nimesulide. Clin Pharmacokinet. 1998 Oct;35(4):247-74. doi: 10.2165/00003088-199835040-00001.
Abstract. Nimesulide is a selective COX-2 inhibitor used in a variety of inflammatory, pain and fever states. After healthy volunteers received oral nimesulide 100 mg in tablet, granule or suspension form the drug was rapidly and extensively absorbed. Mean peak concentrations (Cmax) of 2.86 to 6.50 mg/L were achieved within 1.22 to 2.75 hours of administration. The presence of food did not reduce either the rate or extent of nimesulide absorption. When nimesulide was administered in the suppository form, the Cmax was lower and occurred later than after oral administration; the bioavailability of nimesulide via suppository ranged from 54 to 64%, relative to that of orally administered formulations.....
Kshirsagar NA, Bachhav SS. Nimesulide controversy: a comparison of EU and Indian scenario. Int J Risk Saf Med. 2013;25(4):239-46. doi: 10.3233/JRS-130602.
Abstract. To compare and evaluate, benefit and risk data for regulatory action, of nimesulide in India and EU.
Kasciuškevičiūtė S, Gumbrevičius G, Vendzelytė A, Ščiupokas A, Petrikonis K, Kaduševičius E. Impact of the World Health Organization Pain Treatment Guidelines and the European Medicines Agency Safety Recommendations on Nonsteroidal Anti-Inflammatory Drug Use in Lithuania: An Observational Study. Medicina (Kaunas). 2018 May 11;54(2):30. doi: 10.3390/medicina54020030.
Abstract. Background and objective: Irrational use of nonsteroidal anti-inflammatory drugs (NSAIDs) is the main cause of adverse effects-associated hospitalizations among all medication groups leading to extremely increased costs for health care.
Pulkkinen M. Nimesulide in dysmenorrhoea. Drugs. 1993;46 Suppl 1:129-33. doi: 10.2165/00003495-199300461-00028.
Abstract. Nimesulide does not affect active intrauterine pressure, as measured using microsensors, or the direction and velocity of the propagation of uterine activity, but nevertheless alleviates pain significantly by 30 minutes after oral administration. In dysmenorrhoeic patients, resting pressure is high only in the fundus.....
Raupp ÍN, Valério Filho A, Arim AL, Muniz ARC, da Rosa GS. Development and Characterization of Activated Carbon from Olive Pomace: Experimental Design, Kinetic and Equilibrium Studies in Nimesulide Adsorption. Materials (Basel). 2021 Nov 12;14(22):6820. doi: 10.3390/ma14226820.
Abstract. This work aims to develop and characterize an activated charcoal from olive pomace, which is an agro-industrial residue, for adsorption of Nimesulide in liquid effluent and to evaluate the adsorption kinetics and equilibrium using experimental design.
Santos BFE, Costa FO, Vasconcelos AMA, Cyrino RM, Cota LOM. Preemptive effects of ibuprofen and nimesulide on postoperative pain control after open flap periodontal surgeries: A randomized placebo-controlled split-mouth clinical trial. J Periodontol. 2022 Feb;93(2):298-307. doi: 10.1002/JPER.20-0887.
Abstract. The aim of this study was to evaluate and compare the analgesic effects of the preemptive administration of ibuprofen and nimesulide in open flap periodontal surgeries.
Bessone F, Hernandez N, Mendizabal M, Ridruejo E, Gualano G, Fassio E, Peralta M, Fainboim H, Anders M, Tanno H, Tanno F, Parana R, Medina-Caliz I, Robles-Diaz M, Alvarez-Alvarez I, Niu H, Stephens C, Colombato L, Arrese M, Reggiardo MV, Ono SK, Carrilho F, Lucena MI, Andrade RJ. Serious liver injury induced by Nimesulide: an international collaborative study. Arch Toxicol. 2021 Apr;95(4):1475-1487. doi: 10.1007/s00204-021-03000-8.
Abstract. We aim to analyze the clinical phenotype, outcome, and histological features of nimesulide-induced liver injury (nimesulide-DILI). We analyzed 57 cases recruited from the Spanish and Latin American DILI registries.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Nimesulide Review Consensus 10 by CarPas (5242 pt) | 2023-Sep-11 11:01 |
| Read the full Tiiip | (Send your comment) |
Nimesulide is a chemical compound, sulphonamide.
It occurs in the form of a yellow powder. Stable. Incompatible with strong oxidising agents.

What it is used for and where
Medicine
Take only under medical supervision
Non-steroidal anti-inflammatory drug (NSAID) with analgesic and antipyretic properties, launched in 1985 in Italy and marketed in more than 50 countries worldwide with relatively low risk of gastrointestinal side effects. Not available in the United States.
Brief history
Nimesulide was reported to be hepatotoxic in 1997 and its use was restricted or banned in 2002 first in Finland and then in Spain due to the high frequency of associated hepatotoxicity (1). However, several studies have downgraded the problem and the European Medicines Agency (2), after an extensive study, recognised its efficacy in
- intense pain
- dysmenorrhoea
- pain from osteoarthritis
- pain from traumatic sprains and tendinitis
taking into account the risk/benefit ratio, only recommended a maximum daily dose for the 100mg dose. Stricter restrictions apply to the 200mg dose.
An important study from 2010 clarifies the risk/benefit ratio comprehensively: "Results suggest that implementation of regulatory actions regarding nimesulide may have prevented 79 admissions for liver damage, but increased admissions for UGIB by 859 cases."
Nimesulide as a preferential inhibitor of cyclooxygenase-2 could play a potential therapeutic role in the management of patients with cerebral ischaemia (4) and, together with silver complexes, a potential and safe agent for the topical treatment of skin cancer in humans (5).
Antidiabetic activity of Nimesulide has also been demonstrated (6).
However, recently, a study by Brazilian, Spanish and Argentinean researchers reiterated, this time negatively, the risk/benefit ratio of Nimesulide (7). Frankly, from the consumer's point of view, it is difficult to make a choice since the same doctors give opposite opinions.
Like all drugs it can cause side effects. Always ask the physician.
For more information: Nimesulide studies
| Appearance | Yellow powder |
| Boiling Point | 442.0±55.0 °C at 760 mmHg |
| Melting Point | 140-146°C |
| Flash Point | 221.1±31.5°C |
| Density | 1.5±0.1 g/cm3 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.638 |
| PSA | 109.60000 |
| LogP | 3.79 |
| Safety |  |
 |  |
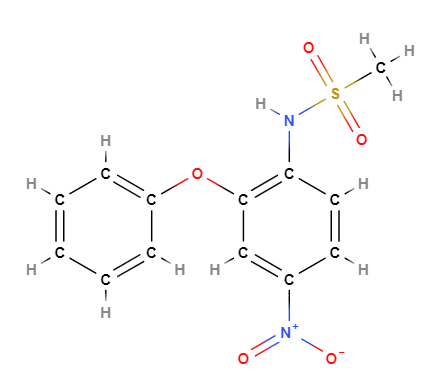 | 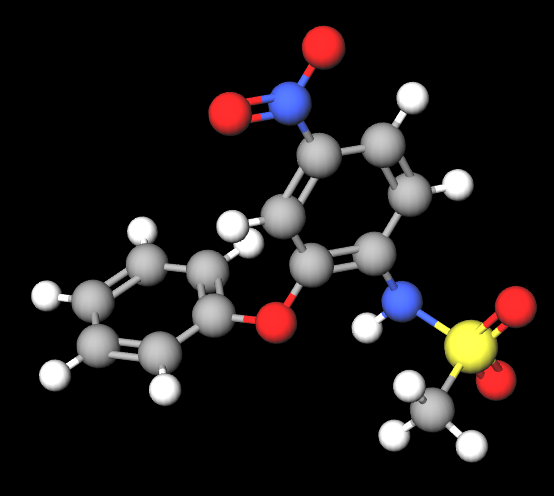 |
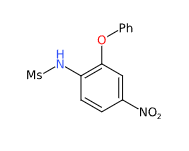
- Molecular Formula C13H12N2O5S
- Linear Formula CH3(CH2)3CH(C2H5)CH2O2CCH2CH(SO3Na)CO2CH2CH(C2H5)(CH2)3CH3
- Molecular Weight 308.31
- Exact Mass 308.046692
- CAS 51803-78-2
- UNII V4TKW1454M
- EC Number 257-431-4
- DSSTox Substance ID DTXSID3037250
- IUPAC N-(4-nitro-2-phenoxyphenyl)methanesulfonamide
- InChI=1S/C13H12N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h2-9,14H,1H3
- InChl Key HYWYRSMBCFDLJT-UHFFFAOYSA-N
- SMILES CS(=O)(=O)NC1=C(C=C(C=C1)[N+](=O)[O-])OC2=CC=CC=C2
- MDL number MFCD00012455
- PubChem Substance ID 24859403
- ChEBI 44445
- RTECS PB0970000
- NCI C29842
Synonyms
- Aulin
- Mesulid
- Flogovital
- Nisulid
- Sulidene
- Methanesulfonamide, N-(4-nitro-2-phenoxyphenyl)-
- Nimed
- Aldoron
- Nise Gel
- Nimedex
- Prestwick_618
- Orthobid
References_________________________________________________________________________
(1) Bessone, F., Fay, F., Fay, O., Vorobioff, J., Passamonti, M. E., Godoy, A., & Tanno, H. (1997, October). Nimesulide hepatotoxicity. In Hepatology (Vol. 26, No. 4, pp. 1417-1417). INDEPENDENCE SQUARE WEST CURTIS CENTER, STE 300, PHILADELPHIA, PA 19106-3399: WB SAUNDERS CO.
(2) epar.P.Nimesulide .EMEA-CPMP-1724-04-en-Final.doc (europa.eu)
(3) Venegoni M, Da Cas R, Menniti-Ippolito F, Traversa G. Effects of the European restrictive actions concerning nimesulide prescription: a simulation study on hepatopathies and gastrointestinal bleedings in Italy. Ann Ist Super Sanita. 2010;46(2):153-7. doi: 10.4415/ANN_10_02_08.
(4) Candelario-Jalil E. Nimesulide as a promising neuroprotectant in brain ischemia: new experimental evidences. Pharmacol Res. 2008 Apr;57(4):266-73. doi: 10.1016/j.phrs.2008.03.003.
(5) Candido TZ, de Paiva REF, Figueiredo MC, de Oliveira Coser L, Frajácomo SCL, Abbehausen C, Cardinalli IA, Lustri WR, Carvalho JE, Ruiz ALTG, Corbi PP, Lima CSP. Silver Nimesulide Complex in Bacterial Cellulose Membranes as an Innovative Therapeutic Method for Topical Treatment of Skin Squamous Cell Carcinoma. Pharmaceutics. 2022 Feb 21;14(2):462. doi: 10.3390/pharmaceutics14020462.
(6) Joharapurkar A, Kshirsagar S, Patel V, Patel M, Savsani H, Jain M. In vivo antidiabetic activity of nimesulide due to inhibition of amino acid transport. Basic Clin Pharmacol Toxicol. 2022 Jan;130(1):35-43. doi: 10.1111/bcpt.13670.
(7) Colombato L, Arrese M, Reggiardo MV, Ono SK, Carrilho F, Lucena MI, Andrade RJ. Serious liver injury induced by Nimesulide: an international collaborative study. Arch Toxicol. 2021 Apr;95(4):1475-1487. doi: 10.1007/s00204-021-03000-8.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2022-05-03 15:25:18 | Chemical Risk: |