![]() Threonine
Threonine
Rating : 7.5
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
10 pts from CarPas
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Threonine studies" about Threonine Review Consensus 10 by CarPas (5242 pt) | 2022-Jun-13 12:39 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Growdon JH, Nader TM, Schoenfeld J, Wurtman RJ. L-threonine in the treatment of spasticity. Clin Neuropharmacol. 1991 Oct;14(5):403-12. doi: 10.1097/00002826-199110000-00003.
Abstract. Preclinical data indicate that the administration of the amino acid L-threonine increases glycine levels in rat spinal cord. In order to investigate glycinergic mechanisms in spasticity, and other signs of the upper motor syndrome, we gave 4.5 and 6.0 g/day of L-threonine to 18 patients with familial spastic paraparesis (FSP) according to a double-blind, crossover protocol. The response to treatment at the end of each 2-week period was based upon three measures: the physician's global impressions; the patients' global impressions; and semiquantitative ratings of strength, muscle tone, DTRs, walking, hopping, and running. Blood and CSF were collected during each treatment period for amino acid analyses. Based upon the severity rating scales, there was a statistically significant (p less than 0.02) decrease in motor impairment and spasticity during L-threonine administration compared to placebo treatment; significant treatment effects were not found on the physician's and patients' global impressions. Plasma and CSF levels of threonine increased significantly during L-threonine treatment but glycine levels did not change. These data indicate that L-threonine significantly suppressed the signs of spasticity even though the benefits were not clinically valuable.
Malinovsky AV. Why Threonine Is an Essential Amino Acid in Mammals and Birds: Studies at the Enzyme Level. Biochemistry (Mosc). 2018 Jul;83(7):795-799. doi: 10.1134/S0006297918070039.
Abstract. In the review, we discuss why threonine is an essential amino acid in mammals and birds based on the pathways of threonine biosynthesis in these two classes of vertebrates.
Kumar Sahoo D, Devi Tulsiyan K, Jena S, Biswal HS. Implication of Threonine-Based Ionic Liquids on the Structural Stability, Binding and Activity of Cytochrome c. Chemphyschem. 2020 Dec 2;21(23):2525-2535. doi: 10.1002/cphc.202000761.
Abstract... , we disclose the molecular level understanding of the structural intactness and reactivity of a model protein cytochrome c (Cyt c) in biocompatible threonine-based ILs with the help of experimental techniques such as isothermal titration calorimetry (ITC), fluorescence spectroscopy, transmission electron microscopy (TEM) as well as molecular docking.
Chapman KP, Courtney-Martin G, Moore AM, Ball RO, Pencharz PB. Threonine requirement of parenterally fed postsurgical human neonates. Am J Clin Nutr. 2009 Jan;89(1):134-41. doi: 10.3945/ajcn.2008.26654.
Abstract. The objective was to determine the parenteral threonine requirement for human neonates by using the minimally invasive indicator amino acid oxidation technique with L-[1-(13)C]phenylalanine as the indicator amino acid.
Rosenthal RG, Vögeli B, Wagner T, Shima S, Erb TJ. A conserved threonine prevents self-intoxication of enoyl-thioester reductases. Nat Chem Biol. 2017 Jul;13(7):745-749. doi: 10.1038/nchembio.2375.
Abstract. We show that a single conserved threonine is essential to suppress the formation of a side product that would otherwise act as a high-affinity inhibitor of the enzyme. Substitution of this threonine with isosteric valine increases side-product formation by more than six orders of magnitude, while decreasing turnover frequency by only one order of magnitude.
Janssen PH. Propanol as an end product of threonine fermentation. Arch Microbiol. 2004 Dec;182(6):482-6. doi: 10.1007/s00203-004-0732-y.
Abstract. Clostridium sp. strain 17cr1 was able to ferment L-threonine to propionate and propanol. Electrons arising in the oxidation of 2-oxobutyrate to propionyl-CoA were apparently used in reductive pathway leading to propanol formation. Part of the propionyl-CoA was used to form propionate in an ATP-forming pathway via a propionate kinase, so that the final ATP yield was 0.5 mol per mol of L-threonine metabolised. Other growth substrates were fermented mainly to acetate and butyrate, and the reductive formation of butyrate, from 2 mol of acetyl-CoA or from crotonate or 3-hydroxybutyrate, was the main route for recycling reduced electron carriers arising during oxidative pathways for most substrates.
van der Sluis M, Schaart MW, de Koning BA, Schierbeek H, Velcich A, Renes IB, van Goudoever JB. Threonine metabolism in the intestine of mice: loss of mucin 2 induces the threonine catabolic pathway. J Pediatr Gastroenterol Nutr. 2009 Jul;49(1):99-107. doi: 10.1097/MPG.0b013e3181a23dbe.
Abstract. Previous studies have shown that the intestine uses a major part of the dietary threonine intake for the synthesis of the structural component of the protective intestinal mucus layer, the secretory mucin Muc2. In this context, the high intestinal demand for dietary threonine probably results from its incorporation into secretory mucins rich in threonine residues. Therefore, we compared threonine utilization in the colon of Muc2 knockout (Muc2-/-) and wild-type (Muc2+/+) mice to investigate the intestinal dietary threonine metabolism in the absence of Muc2, which results in inflammation of the colon.
Moundras C, Bercovici D, Rémésy C, Demigné C. Influence of glucogenic amino acids on the hepatic metabolism of threonine. Biochim Biophys Acta. 1992 Jan 23;1115(3):212-9. doi: 10.1016/0304-4165(92)90056-z.
Abstract. The supplementation of a low-protein diet with L-threonine leads to a marked accumulation of threonine in plasma and liver, whereas increasing dietary protein generally leads to an induction of threonine dehydratase in the liver, hence depressed availability for extrasplanchnic tissues. The aim of the present study was, thus, to further investigate the factors which control the utilization of threonine by the liver.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Threonine Review Consensus 10 by CarPas (5242 pt) | 2022-Dec-24 21:26 |
| Read the full Tiiip | (Send your comment) |
Threonine (α-amino-3-hydroxybutanoic acid) is the α-((R)-1-hydroxy-1-ethyl)-substituted amino acid of proteins. Amino acids play a key metabolic function in the human body and are constituents of proteins. It is a proteinogenic essential amino acid that belongs to the aspartate family, is not synthesised in humans, animals and therefore must be ingested with food. It is also a residual part of proteins such as elastin, collagen, tooth enamel. It prevents the accumulation of fat in the liver by metabolising porphyrin.
α-amino acids that have similar physical structures undergo similar changes with regard to solubility in water/ethanol mixtures, and technologies to separate α-amino acids from industrial residues, which may not even be innocuous, are constantly being improved. However, many data on the solubility in water-ethanol and ethanol of some α-amino acids are contradictory or even lacking, and the effects of ethanol on the solubility of amino acids may be different. Overall, the scientific literature considers that α-amino acids do not pose significant problems for human health when taken orally, except in people with certain genetic diseases.
Food safety: amino acid α generally considered safe.
Cosmetic safety: amino acid α generally considered safe when formulated to be non-irritant.
It appears as a white crystalline powder or as beige crystals. Slightly sweet taste. Soluble in formic acid, in water, practically insoluble in ethanol and ether.

What it is used for and where
Agrochemical intermediate, for food, flavours and fragrances.
Medical
Threonine performs the function of incorporating intestinal mucosa into proteins and synthesises secretory glycoproteins, protecting the intestinal barrier.
Food
Used in food supplements or added to food to improve the nutritional value of proteins. As food additives they perform different functions: preservatives, flavour enhancers, food supplements and more.
Cosmetics
Incorporated into skin care products for a nourishing effect on proteins, collagen. Amino acids together with their salts are used in cosmetics as conditioning agents for both hair and skin (e.g. as moisturisers and other similar functions). Moisturisers are different in nature: the best are the natural ones that exploit the mechanism of integration between the ingredient and the skin by moisturising the horny hydrolipid film, i.e. the thin protective layer that covers the epidermis protecting it from harmful external microbes, keeping the skin moisturised and supple and its pH or acidity value between 4 and 6. Then there are the occlusive moisturisers, usually derived from petroleum (Paraffinum, Paraffinum liquidum and others), but also triglycerides, lanolin oil, natural or synthetic waxes, fatty acid esters and others that create an artificial occlusive layer on the stratum corneum of the skin with the advantage of accelerating the protective process but with the disadvantage of preventing the skin's natural transpiration.
Animal feeding
Threonine has important physiological roles in the animal body: it promotes growth, improves immune function and, in animal feed, reduces the protein requirements of livestock and poultry. In practice, its function is to balance dietary amino acids by bringing the ratio of amino acids closer to the ideal protein. It facilitates fattening, egg production and lactation.
Typical commercial product characteristics L-Threonine
| Appearance | White powder |
| Boiling Point | 345.8±32.0°C at 760 mmHg |
| Melting Point | 255°C |
| Flash Point | 162.9±25.1°C |
| Density | 1.3±0.1 g/cm3 |
| Loss on drying | ≤1.0% |
| Water | ≤0.5% |
| Sulphated ash | ≤0.5%/g |
| Residue on ignition | ≤0.03% |
| Total Impurity | ≤0.2% |
| Refraction Index | 1.507 |
| PSA | 83.55000 |
| LogP | -1.23 |
| Vapor Pressure | 0.0±1.7 mmHg at 25°C |
| Safety |  |
 |  |
 |  |
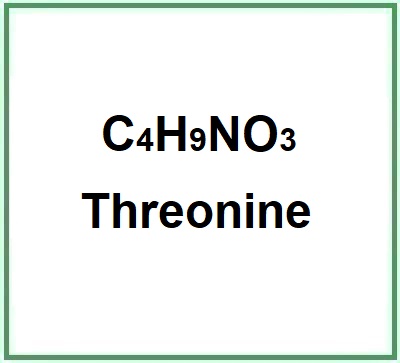
- Molecular Formula C4H9NO3
- Linear Formula CH3CH(OH)CH(NH2)CO2H
- Molecular Weight 119.12
- Exact Mass 119.058243
- CAS 72-19-5
- UNII 2ZD004190S
- EC Number 200-774-1
- DSSTox Substance ID DTXSID2046412
- IUPAC (2S,3R)-2-amino-3-hydroxybutanoic acid
- InChI=1S/C4H9NO3/c1-2(6)3(5)4(7)8/h2-3,6H,5H2,1H3,(H,7,8)/t2-,3+/m1/s1
- InChl Key AYFVYJQAPQTCCC-GBXIJSLDSA-N
- SMILES CC(C(C(=O)O)N)O
- MDL number MFCD00064270
- PubChem Substance ID 24900544
- ChEBI 16857
- JECFA 2119
- Beilstein 1721646
- RXCUI 10524
- NSC 760118
- RTECS XO8590000
- FEMA 4710
- NCI C29602
- eCl@ss 32160406
- NACRES NA.26
Synonyms
- L-Threonine
- (2S,3R)-2-Amino-3-hydroxybutyric acid
- L-alpha-Amino-beta-hydroxybutyric acid
- L-thr
- L-2-Amino-3-hydroxybutyric acid
- Threoninum
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2022-06-13 09:37:16 | Chemical Risk: |