![]() Histidine hcl
Histidine hcl
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
10 pts from CarPas
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "L-Histidine studies" about Histidine hcl Review Consensus 10 by CarPas (5242 pt) | 2022-Jun-21 16:38 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Zhang L, Yadav S, John Wang Y, Mozziconacci O, Schӧneich C. Dual Effect of Histidine on Polysorbate 20 Stability: Mechanistic Studies. Pharm Res. 2018 Jan 16;35(2):33. doi: 10.1007/s11095-017-2321-1.
Abstract. The stability of PS20 in L-His buffer was compared with that in acetate buffer. Forced oxidation of PS20 in these two buffer systems was initiated by a free radical generator, 2,2'-azobis (2-amidinopropane) hydrochloride (AAPH), while accelerated stability tests were carried out at 40°C. Ultra-performance liquid chromatography mass spectrometry was utilized to monitor intact PS20 and to analyze degradation products.
Vangrysperre W, Ampe C, Kersters-Hilderson H, Tempst P. Single active-site histidine in D-xylose isomerase from Streptomyces violaceoruber. Identification by chemical derivatization and peptide mapping. Biochem J. 1989 Oct 1;263(1):195-9. doi: 10.1042/bj2630195.
Abstract. Group-specific chemical modifications of D-xylose isomerase from Streptomyces violaceruber indicated that complete loss of activity is fully correlated with the acylation of a single histidine. Active-site protection, by the ligand combination of xylitol plus Mg2+, completely blocked diethyl pyrocarbonate derivatization of this particular residue [Vangrysperre, Callens, Kersters-Hilderson & De Bruyne (1988) Biochem. J. 250, 153-160]. Differential peptide mapping between D-xylose isomerase, which has previously been treated with diethyl pyrocarbonate in the presence or absence of xylitol plus Mg2+, allowed specific isolation and sequencing of a peptide containing this active-site histidine. For this purpose we used two essentially new techniques: first, a highly reproducible peptide cleavage protocol for protease-resistant, carbethoxylated proteins with guanidinium hydrochloride as denaturing agent and subtilisin for proteolysis; and second, reverse-phase liquid chromatography with dual-wavelength detection at 214 and 238 nm, and calculation of absorbance ratios. It allowed us to locate the single active-site histidine at position 54 in the primary structure of Streptomyces violaceoruber D-xylose isomerase. The sequence around this residue is conserved in D-xylose isomerases from a diversity of micro-organisms, suggesting that this is a structurally and/or functionally essential part of the molecule.
Vilchiz VH, Norman RE, Chang SC. L-histidine methyl ester dihydrochloride. Acta Crystallogr C. 1996 Mar 15;52 ( Pt 3):696-8. doi: 10.1107/s0108270195013308.
Abstract. The title compound, C7H13N3O2(2+).2Cl-, has distances and angles quite similar to those of histidine hydrochloride monohydrate [Donohue & Caron (1964). Acta Cryst. 17, 1178-1180], except for the distances within the ester functionality.
Kolhe P, Amend E, Singh SK. Impact of freezing on pH of buffered solutions and consequences for monoclonal antibody aggregation. Biotechnol Prog. 2010 May-Jun;26(3):727-33. doi: 10.1002/btpr.377.
Abstract. Freezing of biologic drug substance at large scale is an important unit operation that enables manufacturing flexibility and increased use-period for the material. Stability of the biologic in frozen solutions is associated with a number of issues including potentially destabilizing pH changes.
Johnson RP, Uthaman S, John JV, Lee HR, Lee SJ, Park H, Park IK, Suh H, Kim I. Poly(PEGA)-b-poly(L-lysine)-b-poly(L-histidine) Hybrid Vesicles for Tumoral pH-Triggered Intracellular Delivery of Doxorubicin Hydrochloride. ACS Appl Mater Interfaces. 2015 Oct 7;7(39):21770-9. doi: 10.1021/acsami.5b05338.
Abstract. A series of poly(ethylene glycol) methyl ether acrylate-block-poly(L-lysine)-block-poly(L-histidine) [p(PEGA)30-b-p(Lys)25-b-p(His)n] (n = 25, 50, 75, 100) triblock copolypeptides were designed and synthesized for tumoral pH-responsive intracellular release of anticancer drug doxorubicin hydrochloride (Dox).
Johnson RP, Chung CW, Jeong YI, Kang DH, Suh H, Kim I. Poly(L-histidine)-tagged 5-aminolevulinic acid prodrugs: new photosensitizing precursors of protoporphyrin IX for photodynamic colon cancer therapy. Int J Nanomedicine. 2012;7:2497-512. doi: 10.2147/IJN.S29582.
Abstract. 5-Aminolevulinic acid (ALA) and its derivatives have been widely used in photodynamic therapy. The main drawback associated with ALA-based photodynamic therapy (ALA-PDT) and ALA fluorescence diagnosis results from the hydrophilic nature of ALA and lack of selectivity for tumor versus nontumor cells. The application of certain triggers, such as pH, into conventional sensitizers for controllable (1)O(2) release is a promising strategy for tumor-targeted treatment.
Andi B, West AH, Cook PF. Stabilization and characterization of histidine-tagged homocitrate synthase from Saccharomyces cerevisiae. Arch Biochem Biophys. 2004 Jan 15;421(2):243-54. doi: 10.1016/j.abb.2003.11.005.
Abstract. Histidine-tagged homocitrate synthase from Saccharomyces cerevisiae was purified to about 98% using a Ni-NTA resin and stabilized using a combination of 100 mM guanidine hydrochloride, 100 mM alpha-cyclodextrin, and 600 mM ammonium sulfate. The enzyme was assayed using dichlorophenol indophenol (DCPIP) as an oxidant to oxidize the CoASH produced in the reaction.
Considine KL, Stefanidis L, Grozinger KG, Audie J, Alper BJ. Efficient synthesis of α-fluoromethylhistidine di-hydrochloride and demonstration of its efficacy as a glutathione S-transferase inhibitor. Bioorg Med Chem Lett. 2017 Mar 15;27(6):1335-1340. doi: 10.1016/j.bmcl.2017.02.024.
Abstract. Histidine decarboxylase (HDC) is an enzyme that converts histidine to histamine. Inhibition of HDC has several medical applications, and HDC inhibitors are of considerable interest for the study of histidine metabolism.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Histidine hcl Review Consensus 10 by CarPas (5242 pt) | 2023-Sep-08 11:59 |
| Read the full Tiiip | (Send your comment) |
Histidine hcl o L-Histidine hydrochloride is a chemical compound, an amino acid, hydrochloride salt of histidine, synthesised industrially by fermentation with Escherichia coli. It is found in many parts of the human body, e.g. in the organic matrix of dentin/enamel and helps metabolise trace elements such as Zinc, Iron, Manganese, Copper.
The name describes the structure of the molecule
- "Histidine" refers to the amino acid histidine, one of the essential amino acids that the human body cannot synthesize and therefore must obtain through the diet.
- "HCl" stands for hydrochloride. This indicates that histidine has been combined with hydrochloric acid, forming the hydrochloride salt of the amino acid.
Description of raw materials used in production
- Histidine. An essential amino acid with an imidazole functional group. It plays a crucial role in various proteins and enzymes.
- Hydrochloric Acid (HCl). A strong acid commonly used in chemistry.
Step-by-step summary of industrial chemical synthesis process
Biosynthesis.
- Histidine is biosynthesized in bacteria, fungi, and plants from the sugar phosphate named 5-phosphoribosyl-1-pyrophosphate (PRPP). It undergoes several enzymatic transformations leading to the production of histidine.
Chemical Synthesis.
- While more common in research settings than commercial ones, it is possible to synthesize histidine through various chemical reactions.
Combination with Hydrochloric Acid.
- Histidine is then combined with hydrochloric acid to form histidine hydrochloride, or Histidine HCl.
It occurs as a fine white powder.

What it is used for and where
It is an α-amino acid.
Amino acids play a key metabolic function in the human body and are constituents of proteins.
As food additives they perform different functions: preservatives, flavour enhancers, food supplements and more.
Amino acids together with their salts are used in cosmetics as conditioning agents for both hair and skin (e.g. as moisturisers and other similar functions). Moisturisers are different in nature: the best are the natural ones that exploit the mechanism of integration between the ingredient and the skin by moisturising the horny hydrolipid film, i.e. the thin protective layer that covers the epidermis protecting it from harmful external microbes, keeping the skin moisturised and supple and its pH or acidity value between 4 and 6. Then there are the occlusive moisturisers, usually derived from petroleum (Paraffinum, Paraffinum liquidum and others), but also triglycerides, lanolin oil, natural or synthetic waxes, fatty acid esters and others that create an artificial occlusive layer on the stratum corneum of the skin with the advantage of accelerating the protective process but with the disadvantage of preventing the skin's natural transpiration.
α-amino acids that have similar physical structures undergo similar changes with regard to solubility in water/ethanol mixtures, and technologies to separate α-amino acids from industrial residues, which may not even be innocuous, are constantly being improved. However, many data on the solubility in water-ethanol and ethanol of some α-amino acids are contradictory or even lacking, and the effects of ethanol on the solubility of amino acids may be different. Overall, the scientific literature considers that α-amino acids do not pose significant problems for human health when taken orally, except in people with certain genetic diseases.
Food safety: amino acid α generally considered safe.
Cosmetic safety: amino acid α generally considered safe when formulated to be non-irritant.
Animal feeding
Nutritional additive The active ingredient is L-histidine. Flavouring compound for feed of all animal species, to supplement the diet in adequate quantities to cover requirements, depending on species, physiological state of the animal, performance level, environmental conditions, background amino acid composition of the non-supplemented diet and the status of certain essential trace elements (1).
Medical
Excipient used in parenteral products to stabilise biotherapy. Recent studies have highlighted the usefulness of pH-sensitive micelles also containing Histidine as photosensitising carriers to improve photodynamic cancer therapy (2). It protects brain cells where it formulates a compound called metallothionein.
Cosmetics
Used as a chelating agent in personal care products and as an adjuvant in improving the treatment of skin roughness.
The most relevant studies on this ingredient have been selected with a summary of their contents:
L-Histidine hydrochloride studies
Typical commercial product characteristics L-histidine dihydrochloride
| Appearance | White powder |
| pH | 3.5-4.5 (100g/l, H2O, 20℃) |
| Boiling Point | 458.9ºC at 760 mmHg |
| Flash Point | 231.3ºC |
| Melting point | 254 °C |
| Density | 1.49 g/cm3 |
| PSA | 92.00000 |
| LogP | 0.86640 |
| Vapor pressure | <1 hPa (20°C) |
| Water solubility | 169.9 g/L (20 ℃) |
| Shelf life | 2 Years |
| Storage | 2-8°C |
 |  |
 |  |
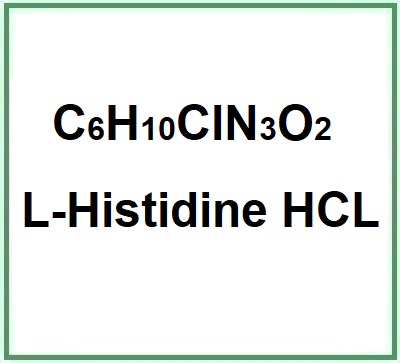
- Molecular Formula C6H10ClN3O2 C6H9N3O2 · HCl
- Molecular Weight 191.61
- Exact Mass 191.046158
- CAS 645-35-2 1007-42-7
- UNII 1D5Q932XM6
- EC Number 211-438-9 213-754-2
- DSSTox Substance ID DTXSID3020700
- IUPAC (2S)-2-amino-3-(1H-imidazol-5-yl)propanoic acid;hydrochloride
- InChI=1S/C6H9N3O2.ClH/c7-5(6(10)11)1-4-2-8-3-9-4;/h2-3,5H,1,7H2,(H,8,9)(H,10,11);1H/t5-;/m0./s1
- InChl Key QZNNVYOVQUKYSC-JEDNCBNOSA-N
- SMILES C1=C(NC=N1)CC(C(=O)O)N.Cl
- MDL number MFCD00064556
- PubChem Substance ID 329756297
- RXCUI 1309689
- Beilstein 4168261
- RTECS MS3116100
- eCl@ss 32160406
- NCACRES NA.24
Synonyms
- (2S)-2-amino-3-(1H-imidazol-5-yl)propanoic acid;hydrochloride
- Histidine-L-HCl
- L-Histidine hydrochloride
- L-Histidine, monohydrochloride
References_____________________________________________________________________
(1) EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP), Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Fašmon Durjava M, Kouba M, López-Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Herman L, Anguita M, Galobart J, Pettenati E, Tarrés-Call J. Safety and efficacy of a feed additive consisting of l-histidine monohydrochloride monohydrate produced using Escherichia coli NITE SD 00268 for all animal species (Kyowa Hakko Europe GmbH). EFSA J. 2021 May 31;19(5):e06622. doi: 10.2903/j.efsa.2021.6622.
(2) Debele TA, Mekuria SL, Tsai HC. A pH-sensitive micelle composed of heparin, phospholipids, and histidine as the carrier of photosensitizers: Application to enhance photodynamic therapy of cancer. Int J Biol Macromol. 2017 May;98:125-138. doi: 10.1016/j.ijbiomac.2017.01.103.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2023-09-08 11:50:16 | Chemical Risk: |