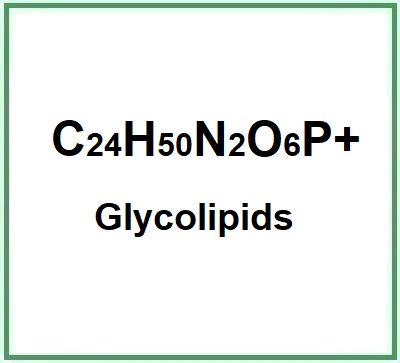![]() Glycolipids
Glycolipids
Rating : 8
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Antifungal (1) Antimicrobial (1)10 pts from CarPas
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Glycolipid studies" about Glycolipids Review Consensus 10 by CarPas (5242 pt) | 2022-Jun-26 12:57 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Anderson BL, Teyton L, Bendelac A, Savage PB. Stimulation of natural killer T cells by glycolipids. Molecules. 2013 Dec 16;18(12):15662-88. doi: 10.3390/molecules181215662.
Abstract. Natural killer T (NKT) cells are a subset of T cells that recognize glycolipid antigens presented by the CD1d protein. The initial discovery of immunostimulatory glycolipids from a marine sponge and the T cells that respond to the compounds has led to extensive research by chemists and immunologists to understand how glycolipids are recognized, possible responses by NKT cells, and the structural features of glycolipids necessary for stimulatory activity. The presence of this cell type in humans and most mammals suggests that it plays critical roles in antigen recognition and the interface between innate and adaptive immunity.
Stocker BL, Timmer MS. Chemical tools for studying the biological function of glycolipids. Chembiochem. 2013 Jul 8;14(10):1164-84. doi: 10.1002/cbic.201300064.
Abstract. The purpose of this review is to present the key approaches that can be used for the development of glycolipid probes, organised according to application, and also to discuss the limitations of such strategies, which include the nontrivial task of ensuring that the probe does not adversely influence the biological activity of the parent compound.
van Dijk JHM, van Hooij A, Groot LM, Geboers J, Moretti R, Verhard-Seymonsbergen E, de Jong D, van der Marel GA, Corstjens PLAM, Codée JDC, Geluk A. Synthetic Phenolic Glycolipids for Application in Diagnostic Tests for Leprosy. Chembiochem. 2021 Apr 16;22(8):1487-1493. doi: 10.1002/cbic.202000810.
Abstract. We describe herein the assembly of a set of phenolic glycolipid Iglycans carrying the characteristic phenol aglycon and featuring different methylation patterns.
Warnecke D, Heinz E. Glycolipid headgroup replacement: a new approach for the analysis of specific functions of glycolipids in vivo. Eur J Cell Biol. 2010 Jan;89(1):53-61. doi: 10.1016/j.ejcb.2009.10.009.
Abstract... This new approach requires the exchange of a lipid glycosyltransferase in an organism by a heterologous glycosyltransferase having a different headgroup specificity, e.g. the substitution of a galactosyltransferase by a glucosyltransferase. The resulting transgenic organism produces a novel glycolipid which differs from that of the native organism not in proportion, but only in structural details of its headgroup...
Roelants SL, Ciesielska K, De Maeseneire SL, Moens H, Everaert B, Verweire S, Denon Q, Vanlerberghe B, Van Bogaert IN, Van der Meeren P, Devreese B, Soetaert W. Towards the industrialization of new biosurfactants: Biotechnological opportunities for the lactone esterase gene from Starmerella bombicola. Biotechnol Bioeng. 2016 Mar;113(3):550-9. doi: 10.1002/bit.25815.
Abstract. Although sophorolipids (SLs) produced by S. bombicola are a real showcase for the industrialization of microbial biosurfactants, some important drawbacks are associated with this efficient biological process, e.g., the simultaneous production of acidic and lactonic SLs. Depending on the application, there is a requirement for the naturally produced mixture to be manipulated to give defined ratios of the components.
Jala RCR, Vudhgiri S, Kumar CG. A comprehensive review on natural occurrence, synthesis and biological activities of glycolipids. Carbohydr Res. 2022 Jun;516:108556. doi: 10.1016/j.carres.2022.108556.
Abstract...this review significantly summarises the biological evaluation of a diverse class of glycolipids isolated and/or synthesized either by partial or total synthesis.
Layre E. Targeted Lipidomics of Mycobacterial Lipids and Glycolipids. Methods Mol Biol. 2021;2314:549-577. doi: 10.1007/978-1-0716-1460-0_24.
Abstract. This chapter describes an HPLC-MS methodology allowing the simultaneous screening of more than 20 of the lipid families produced by mycobacteria and provides recommendations to analyze the generated data given the state-of-the-art.
Rog T, Vattulainen I, Karttunen M. Modeling glycolipids: take one. Cell Mol Biol Lett. 2005;10(4):625-30.
Abstract. Molecular dynamics simulations of glycolipid bilayers consisting of 1,2-di-O-palmitoyl-3-O-beta-D-glucosyl-sn-glycerol were performed using five different force field parameterizations. Comparing the results with experimental data revealed that only the all-atom model correctly reproduces both the phase behavior and the surface area per lipid. Only one of the united atom models studied reproduces the correct phase behavior.
Pask-Hughes RA, Shaw N. Glycolipids from some extreme thermophilic bacteria belonging to the genus Thermus. J Bacteriol. 1982 Jan;149(1):54-8. doi: 10.1128/jb.149.1.54-58.1982.
Abstract. The lipids of Thermus aquaticus YT1, Thermus thermophilus HB8, Thermus sp. strains H and J (from Icelandic hot springs), and Thermus sp. strain NH (from domestic hot water) have been investigated. Each strain contained two major components, a glycolipid and a glycophospholipid, which have been isolated and analyzed. All of the strains contained as the principal component (41 to 57% of total lipid) a diacyldiglycosyl-(N-acyl)glycosaminylglucosylglycerol, but the five glycolipids differed in carbohydrate composition. The glycophospholipid appeared to be identical in each strain and contained an N-acylglucosamine residue. The principal fatty acids were C15 and C17 branched-chain compounds. This unique polar lipid composition should be of value in the classification of other thermophiles in the genus Thermus. The exceptionally high carbohydrate content of the lipids of these extreme thermophiles may be of significance in relation to the molecular basis of thermophily.
Inès M, Dhouha G. Glycolipid biosurfactants: Potential related biomedical and biotechnological applications. Carbohydr Res. 2015 Oct 30;416:59-69. doi: 10.1016/j.carres.2015.07.016.
Abstract. Their antiviral and antitumor effects enable their use in pharmaceutic as therapeutic agents. Also, glycolipids can inhibit the bioadhesion of pathogenic bacteria enabling their use as anti-adhesive agents and for disruption of biofilm formation and can be used in cosmetic industry.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Glycolipids Review Consensus 10 by CarPas (5242 pt) | 2023-Apr-08 16:13 |
| Read the full Tiiip | (Send your comment) |
Glycolipids are biologically important , complex mixtures with low molecular weight, in natural sources, a class of lipids (about 300 oligosaccharide groups) found in brain and other nerve tissue. They are involved in many biological events as receptors of biosignals, modulating receptor function. In the plant kingdom they are found in the biomembranes of fungi, bacteria, animals, in photosynthetic organelles in plants and algae where they are essential for maintaining optimal photosynthesis efficiency. They are obtained from chicken egg yolk, bovine spinal cord, microbacteria and yeasts.
They are presented in the form of a white powder.

There are various glycolipids obtained from yeast microorganisms (1):
Trehalose lipids (derived from Rhodococcus erythropoli), Mannosilleretritol lipids (derived from Pseudozyma aphidis, Pseudozyma antarctica), Cellobiose lipids (derived from Pseudozyma flocculosa,Ustilago maydis), Sophorolipids (derived from Rhodotorula bogoriensis, Starmerella bombicola, Candida apicola).
What they are used for and where they are used
Biotensioactives, which contain phosphocholine and phosphoethanolamine, play a role as secondary metabolites. They are used industrially, but still have a high cost of production and a relatively low yield, so their use is still rather limited.
Medical
Glycolipids play an important role in the development and maintenance of nerve tissue (2) with some antifungal, antimicrobial and antiviral properties. They can form pores and destabilise the biological membrane by acting as haemolytic agents (3).
Food
Used as antibacterial for food preservation. Ingredient included in the list of European food additives as E246, preservative.
Cosmetics
Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
Included in formulations as bio-preservatives with anti-fungal properties to prevent product deterioration due to pathogenic bacteria Due to their anti-adhesive properties that can interrupt biofilm formation, they are also included in toothpastes.
For more information:
| Appearance | White powder |
| PSA | 144.36000 |
| LogP | 9.53 |
| Storage | −20°C |
 |  |
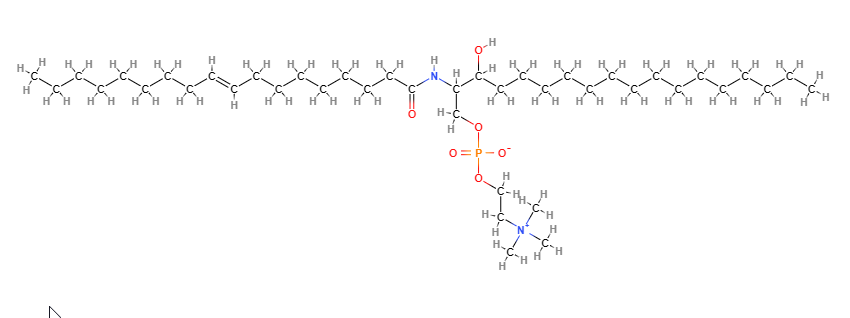 | 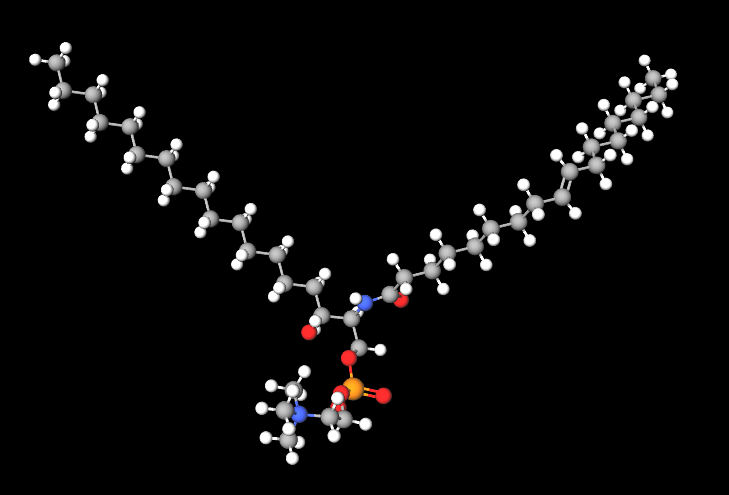 |
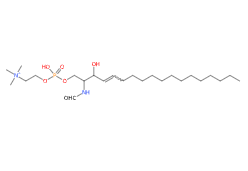
- Molecular Formula C41H84N2O6P C24H50N2O6P+
- Molecular Weight 493.6
- CAS 85187-10-6
- UNII 25WE4JC35O
- EC Number 286-097-2
- DSSTox Substance ID
- IUPAC 2-[[(E,2S,3R)-2-formamido-3-hydroxyoctadec-4-enoxy]-hydroxyphosphoryl]oxyethyl-trimethylazanium
- InChI=1S/C24H49N2O6P/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-24(28)23(25-22-27)21-32-33(29,30)31-20-19-26(2,3)4/h17-18,22-24,28H,5-16,19-21H2,1-4H3,(H-,25,27,29,30)/p+1/b18-17+/t23-,24+/m0/s1
- InChl Key LRYZPFWEZHSTHD-HEFFAWAOSA-O
- SMILES CCCCCCCCCCCCCC=CC(C(COP(=O)(O)OCC[N+](C)(C)C)NC=O)O
- MDL number MFCD00132361
- PubChem Substance ID
- NACRES NA.25
- CCRIS 7516
Synonyms
- Sphingomyelins
- N-acyl-sphing-4-enine-1-phosphocholine
- Ethanaminium, 2-[[hydroxy[[(2S,3S,4E)-3-hydroxy-2-[(1-oxohexadecyl)amino]-4-octadecen-1-yl]oxy]phosphinyl]oxy]-N,N,N-trimethyl-, inner salt
- N-acil-4-sfingenil-1-O-fosforilcolina
References______________________________________________________________________
(1) Jezierska S, Claus S, Van Bogaert I. Yeast glycolipid biosurfactants. FEBS Lett. 2018 Apr;592(8):1312-1329. doi: 10.1002/1873-3468.12888.
(2) Furukawa K, Okuda T, Furukawa K. Roles of glycolipids in the development and maintenance of nervous tissues. Methods Enzymol. 2006;417:37-52. doi: 10.1016/S0076-6879(06)17004-4.
(3) Inès M, Dhouha G. Glycolipid biosurfactants: Potential related biomedical and biotechnological applications. Carbohydr Res. 2015 Oct 30;416:59-69. doi: 10.1016/j.carres.2015.07.016.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2022-10-16 21:36:09 | Chemical Risk: |