![]() Ketoprofen
Ketoprofen
Rating : 7.5
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Anti-inflammatory (1) Analgesic (1)Cons:
Avoid excessive amounts (1)10 pts from Street82
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Ketoprofen studies" about Ketoprofen Review Consensus 10 by Street82 (2970 pt) | 2022-Jul-03 12:14 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Avouac B, Teule M. Ketoprofen: the European experience. J Clin Pharmacol. 1988 Dec;28(s1):S2-7. doi: 10.1002/j.1552-4604.1988.tb05972.x.
Abstract. An overview of European experience with ketoprofen, a nonsteroidal anti-inflammatory drug (NSAID) with analgesic properties, from the time of its marketing in 1973 until the present is presented. Orally administered ketoprofen (200 mg/day) has been proven effective in treating rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis. Furthermore, several alternate dosage forms, including intramuscular injection for relief of acutely painful conditions, suppositories, two slow-release forms (a sustained-release tablet [IBP 200] and a controlled-release capsule [Oruvail] ), and a topical gel for local treatment of certain superficial conditions and minor rheumatologic disease are available. The safety of ketoprofen has also been proven in several European postmarketing surveillance studies and more importantly, by French and British drug monitoring data. Ketoprofen was rated as one of the safest NSAIDs available in the United Kingdom (UK) by the Committee on the Safety of Medicine in 1986. For incidence of gastrointestinal complaints per million prescriptions, ketoprofen ranked seventh among 19 NSAIDs in its first five years of marketing in the UK. Ketoprofen has been associated with a very low incidence of serious renal, hepatic, or cutaneous reactions. Thus ketoprofen, in 15 years of marketed use in Europe, has proven to be an effective anti-inflammatory and analgesic agent with an excellent safety profile and several convenient dosage forms.
Cailleteau JG. Ketoprofen in dentistry: a pharmacologic review. Oral Surg Oral Med Oral Pathol. 1988 Nov;66(5):620-4. doi: 10.1016/0030-4220(88)90386-6.
Abstract. The pharmacology, clinical dental studies, and the relevance to dental applications of ketoprofen, a recent addition to the group of nonsteroidal anti-inflammatory drugs, are reviewed. In addition to inhibiting the formation of prostaglandins, ketoprofen may also suppress leukotrienes and further limit inflammation. Clinically, it appears that ketoprofen compares favorably with many of the nonsteroidal drugs now in use. Recently approved for use in mild to moderate pain, ketoprofen's rapid onset of action, patent analgesic properties, and minimal side effects are particularly appealing.
Coaccioli S. Ketoprofen 2.5% gel: a clinical overview. Eur Rev Med Pharmacol Sci. 2011 Aug;15(8):943-9.
Abstract. Ketoprofen (KP), a nonsteroidal anti-inflammatory drug (NSAID), possesses analgesic, antipyretic and anti-inflammatory properties. Oral KP is widely used in musculoskeletal pain and inflammation in muscles and joints, including arthritis pain, osteoarthritis, stiffness of the joints, soft tissue rheumatism, and sports injuries.
Kantor TG. Ketoprofen: a review of its pharmacologic and clinical properties. Pharmacotherapy. 1986 May-Jun;6(3):93-103. doi: 10.1002/j.1875-9114.1986.tb03459.x.
Abstract. ... Today the drug is available in about 80 countries and has recently been approved in the United States for treatment of rheumatoid arthritis and osteoarthritis. The therapeutic experience with ketoprofen is estimated to have exceeded 3 million patient-years. Double-blind trials have established its therapeutic equivalence with aspirin, indomethacin, and ibuprofen in rheumatoid arthritis and with aspirin in osteoarthritis. Ketoprofen has a short half-life, a simple metabolism, and a broad therapeutic window, and does not accumulate with multiple doses. These features contribute to a rapid onset of action, flexible dosing, and a reliable tolerance profile.
Raffaini G, Catauro M. Surface Interactions between Ketoprofen and Silica-Based Biomaterials as Drug Delivery System Synthesized via Sol-Gel: A Molecular Dynamics Study. Materials (Basel). 2022 Apr 8;15(8):2759. doi: 10.3390/ma15082759.
Abstract. Biomaterial-based drug delivery systems for a controlled drug release are drawing increasing attention thanks to their possible pharmaceutical and biomedical applications. It is important to control the local administration of drugs, especially when the drug exhibits problems diffusing across biological barriers. Thus, in an appropriate concentration, it would be released in situ, reducing side effects due to interactions with the biological environment after implantation. A theoretical study based on Molecular Mechanics and Molecular Dynamics methods is performed to investigate possible surface interactions between the amorphous SiO2 surface and the ketoprofen molecules, an anti-inflammatory drug, considering the role of drug concentration.
Sychev DA, Morozova TE, Shatskiy DA, Shikh NV, Shikh EV, Andrushchyshina TB, Lukina MV, Kachanova AA, Sozaeva ZA, Abdullaev SP, Denisenko NP, Ryzhikova KA. Effect of CYP2C9, PTGS-1 and PTGS-2 gene polymorphisms on the efficiency and safety of postoperative analgesia with ketoprofen. Drug Metab Pers Ther. 2022 Jun 15. doi: 10.1515/dmpt-2021-0222.
Abstract. Patients undergoing cardiac surgery develop post-sternotomy pain syndrome. The aim of this study was evaluation of the influence of CYP2C9, PTGS-1 and PTGS-2 genes polymorphisms on the efficacy and safety of postoperative analgesia with ketoprofen in patients with coronary artery disease after cardiac surgery.
Soare AC, Meltzer V, Colbea C, Stanculescu I, Pincu E. Compatibility of Drotaverine Hydrochloride with Ibuprofen and Ketoprofen Nonsteroidal Anti-Inflammatory Drugs Mixtures. Materials (Basel). 2022 Feb 8;15(3):1244. doi: 10.3390/ma15031244.
Abstract. The compatibility of Ibuprofen, Ketoprofen, and Drotaverine Hydrochloride was investigated using differential scanning calorimetry (DSC), X-ray diffraction (XRD), and Fourier-Transform Infrared spectroscopy (FTIR). Solid-liquid equilibrium (SLE) phase diagrams for the binary systems of active pharmaceutical ingredients were developed and the Tammann diagrams were designed to determine the eutectic compositions. The excess thermodynamic functions GE for the pre-, post-, and eutectic compositions were obtained using the computed activity coefficients data. Results show that drotaverine-based pharmaceutical forms for pain treatment may be obtained at 0.9 respectively 0.8 molar fractions of ibuprofen and ketoprofen which is advantageous because the maximum allowed daily dose of Ibu is about 6 times higher than those of D-HCl and Ket. The obtained eutectics may be a viable option for the treatment of pain associated with cancer therapy.
Loh TY, Cohen PR. Ketoprofen-induced photoallergic dermatitis. Indian J Med Res. 2016 Dec;144(6):803-806. doi: 10.4103/ijmr.IJMR_626_16.
Abstract. Drug-induced photosensitivity reactions are significant adverse effects. Ketoprofen is one of the most common drugs that can cause skin rash in sun-exposed areas. Non-steroidal anti-inflammatory drugs (NSAIDs), such as ketoprofen, are often used for a variety of symptoms, including pain and fever. An understanding of the presentation and clinical course of ketoprofen-induced photosensitivity is necessary to correctly diagnose and manage this condition.
Cantisani C, Grieco T, Faina V, Mattozzi C, Bohnenberger H, Silvestri E, Calvieri S. Ketoprofen allergic reactions. Recent Pat Inflamm Allergy Drug Discov. 2010 Jan;4(1):58-64. doi: 10.2174/187221310789895595.
Abstract. Topical ketoprofen (KP) is widely used because of its anti-inflammatory effect. Parallel with its popular usage, the number of reported cases of ketoprofen-induced photoallergic contact dermatitis has increased. A review of the literature was made to evaluate the spectrum of cross sensitization in patients with ketoprofen-induced photoallergic contact dermatitis using ketoprofen and other structurally similar chemicals and sunscreens, fragrance components, as well as the presence of prolonged photosensitivity related to it. Furthermore, the distinction between true cross-reactivity and concomitant sensitization may be difficult. Therefore, further investigations are needed to gain a more complete understanding of this important topic. This article also reviews some patents related to alternative treatment of musculoskeletal diseases and/or treatment of allergic reactions due to NSAIDs use.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Ketoprofen Review Consensus 10 by Street82 (2970 pt) | 2022-Jul-03 12:20 |
| Read the full Tiiip | (Send your comment) |
Ketoprofene is a chemical compound, a bone-monocarboxylic acid consisting of propionic acid.
It occurs as a white crystalline powder

What it is used for and where
Medical
Ketoprofen has been marketed in France and the UK since 1973. It belongs to the family of non-steroidal anti-inflammatory drugs (NSAIDs) and has anti-inflammatory activities and local analgesic properties, for which it is used to combat pain, swelling, arthritis and fever. It is used in mild to moderate musculoskeletal pain and inflammation of muscles and joints, sports injuries and soft tissue rheumatism. Topical application generally minimises adverse effects, while oral ingestion, as with all NSAIDs, has been found to be associated with gastrointestinal disturbances and other systemic adverse events.
Recently, Ketoprofen salified with L-lysine has been marketed, credited in animal studies with better gastric tolerance in vitro, greater solubility
(Novelli R, Aramini A, Boccella S, Bagnasco M, Cattani F, Ferrari MP, Goisis G, Minnella EM, Allegretti M, Pace V. Ketoprofen lysine salt has a better gastrointestinal and renal tolerability than ketoprofen acid: A comparative tolerability study in the Beagle dog. Biomed Pharmacother. 2022 Jun 27;153:113336. doi: 10.1016/j.biopha.2022.113336. )
Safety
Cases of allergic reactions to ketoprofen have been established (1).
In this study of 900 children, oral ketoprofen was well tolerated when administered up to 3 weeks after surgery (2).
The results of this study show less harmful effects of ketoprofen than indomethacin on the immature intestine and indicate that the intestinal response to this NSAID varies significantly between individuals (3).
| Appearance | White crystalline powder |
| Boiling Point | 431.3±28.0°C at 760 mmHg |
| Melting Point | 93-96°C |
| Flash Point | 228.8±20.5°C |
| Density | 1.2±0.1 g/cm3 |
| PSA | 54.37000 |
| LogP | 2.81 |
| Refraction Index | 1.592 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Safety |  |
 |  |
 |  |
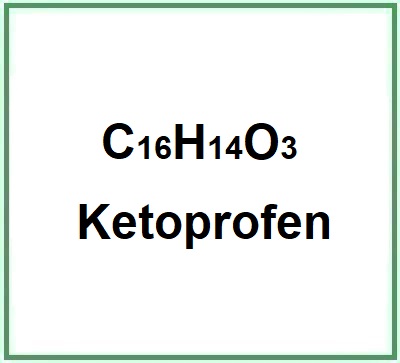
- Molecular Formula C16H14O3
- Molecular Weight 254.28
- Exact Mass 254.094299
- CAS 22071-15-4
- UNII 90Y4QC304K
- EC Number 244-759-8
- DSSTox Substance ID DTXSID6020771
- IUPAC 2-(3-benzoylphenyl)propanoic acid
- InChI=1S/C16H14O3/c1-11(16(18)19)13-8-5-9-14(10-13)15(17)12-6-3-2-4-7-12/h2-11H,1H3,(H,18,19)
- InChl Key DKYWVDODHFEZIM-UHFFFAOYSA-N
- SMILES CC(C1=CC(=CC=C1)C(=O)C2=CC=CC=C2)C(=O)O
- MDL number MFCD00055790
- PubChem Substance ID 57654315
- ChEBI 6128
- NACRES NA.77
- NSC 758144
- RTECS UE7570000
Synonyms
Alrheumum, Aneol, Capisten, Epatec, Orudis, Profenid, Dexal, Keduril, Ketosolan, Kevadon, Oruvail, 3-Benzoyl-alpha-methylbenzeneacetic acid, 2-(3-Benzoylphenyl)propionic acid, 2-(m-Benzoylphenyl)propionic acid, 3-Benzoylhydratropic acid, Benzeneacetic acid, 3-benzoyl-alpha-methyl, m-benzoylhydratropic acid
References___________________________________________________________________
(1) Cantisani C, Grieco T, Faina V, Mattozzi C, Bohnenberger H, Silvestri E, Calvieri S. Ketoprofen allergic reactions. Recent Pat Inflamm Allergy Drug Discov. 2010 Jan;4(1):58-64. doi: 10.2174/187221310789895595.
(2) Kokki H. Ketoprofen pharmacokinetics, efficacy, and tolerability in pediatric patients. Paediatr Drugs. 2010 Oct 1;12(5):313-29. doi: 10.2165/11534910-000000000-00000.
(3) Thibault MP, Tremblay É, Wallace JL, Beaulieu JF. Effect of Ketoprofen and ATB-352 on the Immature Human Intestine: Identification of Responders and Non-responders. J Pediatr Gastroenterol Nutr. 2019 May;68(5):623-629. doi: 10.1097/MPG.0000000000002308.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2022-07-03 10:56:08 | Chemical Risk: |