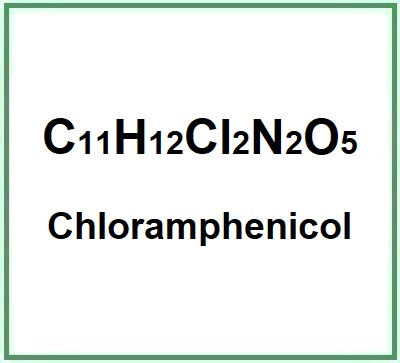
Compendium of the most significant studies with reference to properties, intake, effects.
Fang X, Bennett N, Ierano C, James R, Thursky K. Ophthalmic Antimicrobial Prescribing in Australian Healthcare Facilities. Antibiotics (Basel). 2022 May 12;11(5):647. doi: 10.3390/antibiotics11050647.
Abstract. The National Antimicrobial Prescribing Survey (NAPS) is a web-based, standardized tool, widely adopted in Australian healthcare facilities to assess the reasons for, the quantity of, and the quality of antimicrobial prescribing. It consists of multiple modules tailored towards the needs of a variety of healthcare facilities. Data regarding ophthalmological antimicrobial use from Hospital NAPS, Surgical NAPS, and Aged Care NAPS were analysed. In Hospital NAPS, the most common reasons for inappropriate prescribing were incorrect dose or frequency and incorrect duration. Prolonged duration was also common in Aged Care prescribing: about one quarter of all antimicrobials had been prescribed for greater than 6 months. All three modules found chloramphenicol to be the most prescribed antimicrobial with a high rate of inappropriate prescribing, usually for conjunctivitis.
Bannatyne RM, Cheung R. Chloramphenicol bioassay. Antimicrob Agents Chemother. 1979 Jul;16(1):43-5. doi: 10.1128/AAC.16.1.43.
Abstract. An accurate plate diffusion bioassay for chloramphenicol is described, in which the fast-replicating Beneckea natriegens and 1.5% salt agar are used. Zones of inhibition were well defined after 3 h, and the limit of sensitivity of the method was around 2 mug/ml. The concurrent presence of gentamicin did not influence the assay. The assay is simple to carry out and duplicate assays can be performed with as little as 100 mug of capillary blood.
Field D, Martin D, Witchell L. Ophthalmic chloramphenicol: a review of the literature. Accid Emerg Nurs. 1999 Jan;7(1):13-7. doi: 10.1016/s0965-2302(99)80095-2.
Abstract. The safety of ophthalmic chloramphenicol has been under review in the UK since 1995, when a letter in the British Medical Journal suggested that its use should be discontinued. The writers consult a wide range of American, European and British research from 1950 to the present to reassure readers that ophthalmic chloramphenicol is a demonstrably effective, safe, cost-effective treatment for most superficial eye infections. They advise colleagues to consider any changes in the provision of antibiotic treatment for ophthalmic conditions carefully, as significant changes in practice may incur cost penalties on departmental or practice drug budgets, and may increase the likelihood of treatment failure which could result in patients having to pay second prescription charges. The requirement for nursing practice to be fully accountable, particularly when nurses are supplying drugs using Trust protocols, is emphasized. The article makes explicit the need for nursing practice to be well considered and based on current, credible research, and offers guidelines for nursing practice when ophthalmic chloramphenicol is supplied under protocol.
Aspenström-Fagerlund B, Nordkvist E, Törnkvist A, Wallgren P, Hoogenboom R, Berendsen B, Granelli K. Distribution of chloramphenicol to tissues, plasma and urine in pigs after oral intake of low doses. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2016 Sep;33(9):1411-20. doi: 10.1080/19440049.2016.1209574.
Abstract. Toxic effects of chloramphenicol in humans caused the ban for its use in food-producing animals in the EU. A minimum required performance level (MRPL) was specified for chloramphenicol at 0.3 μg kg(-1) for various matrices, including urine. In 2012, residues of chloramphenicol were found in pig urine and muscle without signs of illegal use. Regarding its natural occurrence in straw, it was hypothesised that this might be the source, straw being compulsory for use as bedding material for pigs in Sweden. Therefore, we investigated if low daily doses of chloramphenicol (4, 40 and 400 μg/pig) given orally during 14 days could result in residues in pig tissues and urine. A dose-related increase of residues was found in muscle, plasma, kidney and urine (showing the highest levels), but no chloramphenicol was found in the liver. At the lowest dose, residues were below the MRPL in all tissues except in the urine. However, in the middle dose, residues were above the MRPL in all tissues except muscle, and at the highest dose in all matrices. This study proves that exposure of pigs to chloramphenicol in doses occurring naturally in straw could result in residues above the MRPL in plasma, kidney and especially urine.
Lietman PS. Chloramphenicol and the neonate--1979 view. Clin Perinatol. 1979 Mar;6(1):151-62.
Abstract. In a therapeutic tragedy perhaps even more widespread than the thalidomide disaster, untold lives were lost between 1949 and 1958 through the administration of inappropriate doses of chloramphenicol to newborn infants. Sensitive assays of blood levels of chloramphenicol now available make it possible to employ this useful antibiotic avoiding its toxic effects. The author reviews accumulated knowledge about chloramphenicol in the newborn infant.
Blogg JR. Chloramphenicol 4. Responsible topical use in ophthalmology. Aust Vet J. 1991 Jan;68(1):8-9. doi: 10.1111/j.1751-0813.1991.tb09829.x.
Abstract. Chloramphenicol has gained widespread use in the topical treatment of ocular infections. The rationale for this use was based on the ability of chloramphenicol to penetrate the cornea and enter the anterior segment, together with its broad spectrum of antimicrobial activity. However, routine use in corneal ulceration or keratitis is not desirable. Hypopyon, when present, is usually sterile. Concerns about human exposure to chloramphenicol and its recent prohibition of use in food-producing animals, raise the need to review its indications and discuss alternatives. The role of chloramphenicol in ocular therapeutics is examined in this article.
Süssmuth R, Haag R, Lingens F. Chloramphenicol resistance of three different flavobacteria. J Antibiot (Tokyo). 1979 Dec;32(12):1293-302. doi: 10.7164/antibiotics.32.1293.
Abstract. The chloramphenicol resistance of some flavobacteria was investigated comparatively. This resistance can be explained either by acetylation of chloramphenicol to O-acetylchloramphenicol via constitutively formed acetyltransferases, followed by cometabolic degradation (strain CB 60), or by limited uptake and total degradation (strain CB 6) by inducible enzymes or by other mechanisms (F. devorans). The mechanisms of resistance, CM-acetylation, CM-degradation and limited uptake are discussed.
Ti TI, Monteiro EH, Lam S, Lee HS. Chloramphenicol concentrations in sera of patients with typhoid fever being treated with oral or intravenous preparation. Antimicrob Agents Chemother. 1990 Sep;34(9):1809-11. doi: 10.1128/AAC.34.9.1809.
Abstract. Serum chloramphenicol and chloramphenicol succinate concentrations in patients given equivalent doses of chloramphenicol base either intravenously or orally for typhoid fever were measured by high-performance liquid chromatography. The mean serum chloramphenicol concentrations were significantly lower in the 11 patients treated with intravenous chloramphenicol succinate than in the 15 patients treated with oral chloramphenicol capsules.
![]() Chloramphenicol
Chloramphenicol