![]() Chlorhexidine
Chlorhexidine
Rating : 7.5
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Oral hygiene. Anti-plaque (1) Antibacterial (1)Cons:
Avoid excessive amounts (1)10 pts from Street82
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Chlorhexidine studies" about Chlorhexidine Review Consensus 10 by Street82 (2970 pt) | 2022-Jul-27 17:48 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Padois K, Bertholle V, Pirot F, Hyunh TT, Rossi A, Colombo P, Falson F, Sonvico F. Chlorhexidine salt-loaded polyurethane orthodontic chains: in vitro release and antibacterial activity studies. AAPS PharmSciTech. 2012 Dec;13(4):1446-50. doi: 10.1208/s12249-012-9872-6.
Abstract. The aim of the present study was to characterize the in vitro release of chlorhexidine from new polymeric orthodontic chains realized with polyurethane loaded with two different chlorhexidine salts: chlorhexidine diacetate or chlorhexidine digluconate. The orthodontic chains constituted of three layers: a middle polyurethane layer loaded with chlorhexidine salt inserted between two layers of unloaded polymer. In vitro release of chlorhexidine diacetate and digluconate from orthodontic chains loaded with 10% or 20% (w/w) chlorhexidine salt was sustained for 42 days and followed Fickian diffusion. The drug diffusion through the polyurethane was found to be dependent not only on chlorhexidine loading, but also on the type of chlorhexidine salt. The antibacterial activity of 0.2% (w/w) chlorhexidine diacetate-loaded orthodontic chain was successfully tested towards clinically isolated biofilm forming ica-positive Staphylococcus epidermidis via agar diffusion test. In conclusion, the chlorhexidine salt-loaded chains could provide an innovative approach in the prevention of oral infections related to the use of orthodontic devices.
Haydari M, Bardakci AG, Koldsland OC, Aass AM, Sandvik L, Preus HR. Comparing the effect of 0.06% -, 0.12% and 0.2% Chlorhexidine on plaque, bleeding and side effects in an experimental gingivitis model: a parallel group, double masked randomized clinical trial. BMC Oral Health. 2017 Aug 18;17(1):118. doi: 10.1186/s12903-017-0400-7.
Abstract. Chlorhexidine is the gold standard of dental plaque prevention. The aim of the present study was to compare the plaque and gingivitis inhibiting effect of commercial products containing 0.2%, 0.12% and 0.06% chlorhexidine in a modified experimental gingivitis model.
Bernardi A, Teixeira CS. The properties of chlorhexidine and undesired effects of its use in endodontics. Quintessence Int. 2015 Jul-Aug;46(7):575-82. doi: 10.3290/j.qi.a33934.
Abstract. The purpose of this article was to review the literature on the properties of chlorhexidine (CHX) and the adverse effects that may occur from its use in endodontics. In addition, adverse effects that may result from its use, such as dark staining of teeth, chemical interaction with sodium hypochlorite and formed flocculate, biologic hazards, and interactions with the filling material were evaluated.
Nezam S, Singh P, Ojha R, Khan SA, Kumari N, Kumari N. Evaluation of the Antimicrobial Activity of Magnetized Water and Its Comparison with Chlorhexidine 0.2% in Young Children for 3 Weeks. J Contemp Dent Pract. 2022 Jan 1;23(1):83-88.
Abstract. The goal of this study was to compare the effects of magnetized water and 0.2% chlorhexidine mouthwash on gingivitis and plaque prevention in children aged 12-15 years for a period of 21 days.
Jeanes A, Bitmead J. Reducing bloodstream infection with a chlorhexidine gel IV dressing. Br J Nurs. 2015 Oct 22-Nov 11;24(19):S14-9. doi: 10.12968/bjon.2015.24.Sup19.S14.
Abstract. The use of vascular access devices (VAD) is common in healthcare provision but there is a significant risk of acquiring an infection. Central venous catheters (CVC) are associated with the highest risk of intravenous catheter-related bloodstream infection (CRBSI). 3M™ Tegaderm™ CHG IV dressing is a semi-permeable transparent adhesive dressing with an integrated gel pad containing chlorhexidine gluconate 2%. This product was reviewed by the National Institute for Health and Care Excellence (NICE) in 2015, recommending that Tegaderm CHG could be used for CVC and arterial line dressings in high-dependency and intensive-care settings. This article discusses issues around CRBSI, interventions to reduce the risk of CRBSI, and the use of Tegaderm CHG dressing.
Lee A, Harlan R, Breaud AR, Speck K, Perl TM, Clarke W, Milstone AM. Blood concentrations of chlorhexidine in hospitalized children undergoing daily chlorhexidine bathing. Infect Control Hosp Epidemiol. 2011 Apr;32(4):395-7. doi: 10.1086/659154.
Abstract. We collected serial blood samples from children in the intensive care unit who underwent daily bathing with 2% chlorhexidine gluconate (CHG)-impregnated cloths. Low concentrations of CHG were detected in a few blood samples, indicating absorption through intact skin. There was no suggestion that CHG accumulated in the blood with repeated exposures.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Chlorhexidine Review Consensus 10 by Street82 (2970 pt) | 2023-Oct-26 18:56 |
| Read the full Tiiip | (Send your comment) |
Chlorhexidine is a cationic bis-biguanide antiseptic, a class of chemically related compounds with proven bacteriostatic and bactericidal effects on Gram-positive and Gram-negative bacteria and against many infectious microbes, including Enterococcus faecalis.
The name describes the structure of the molecule:
- Chlor (Chlorine) is part of the name and indicates the presence of chlorine in the chemical structure of the molecule. Chlorine is known for its disinfecting and antimicrobial properties.
- hexidine refers to the specific chemical structure of the molecule, which includes a hexamethylene ring. This structure is key to its antibacterial properties.
Raw Materials Used in Production.
Chlorhexidine is a synthetic chemical compound, classified as a biguanide. The raw materials for its production include compounds derived from guanidine and chlorine.
Step-by-step Summary of Industrial Production Process.
- Synthesis of the base compound through chemical reactions involving guanidine and chlorine.
- Purification of the compound through various chemical processes.
- Quality control to ensure purity and efficacy.
- Dilution in appropriate solutions, if necessary, for various uses.
It appears in the form of a white, stable powder. Incompatible with strong oxidising agents.

What it is used for and where
Medical
Chlorhexidine has a role in combating, treating and preventing infections of the skin and mucous membranes and for the preservation of eye drops at a concentration of 0.01% w/v. For skin disinfection, chlorhexidine is usually formulated as a 0.5% w/v solution in 70% v/v ethanol and, in combination with detergents, as a 4% w/v surgical scrub.
In dentistry, chlorhexidine is used in the prevention of dental plaque and as a gingivitis inhibitor and binds to the oral mucosa from which it slowly releases. In this study, the clinical and microbiological effects of a mechanical and manual scaling and root planing session and the use of two different chlorhexidine formulations in the treatment of chronic periodontitis were evaluated (1).
For people who wear an internal catheter, it is important that a lubricating gel is used, as this helps to reduce the risk of pain, trauma and infection. Chlorhexidine is an effective antiseptic contained in many catheterisation gels.
In patients undergoing mechanical ventilation, the most effective concentration of chlorhexidine to use to reduce microbial colonisation has proven to be chlorhexidine 1% (2).
During the SARS-CoV-2 outbreak, chlorhexidine showed potential for application as an effective and safe oral rinsing agent to prevent viral transmission (3).
Lysozyme, a lytic enzyme-type protein found in the human body (liver, spleen. saliva) eggs and other animal tissues, a peptide with antimicrobial activity is an effective substitute for chlorhexidine and benzidine in the treatment of acute pharyngitis (4).
Cosmetics
It is a restricted ingredient as V/42 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Substance or ingredient reported: N,N'-bis(4-chlorophenyl)-3,12-diimino-2,4,11,13-tetraazatetradecanediamidine and its digluconate, diacetate and dihydrochloride. Maximum concentration in ready for use preparation 0.3% (as chlorhexidine).
Antimicrobial agent. This ingredient is able to suppress or inhibit the growth and replication of a broad spectrum of microorganisms such as bacteria, fungi and viruses by making the stratum corneum temporarily bactericidal and fungicidal.
Oral care agent. This ingredient can be placed in the oral cavity to improve and/or maintain oral hygiene and health, to prevent or improve a disorder of the teeth, gums, mucous membrane. It provides cosmetic effects to the oral cavity as a protector, cleanser, deodorant.
Preservative. Any product containing organic, inorganic compounds, water, needs to be preserved from microbial contamination. Preservatives act against the development of harmful microorganisms and against oxidation of the product.
Other uses
Cationic surfactant. Organic intermediate.
Commercial Applications
Use in Personal Care and Health Products. Chlorhexidine is commonly used as a disinfectant and antiseptic in dental products, skin cleansers, and skin sanitizers.
Antimicrobial Properties. It has excellent antimicrobial activity against bacteria, viruses, and fungi, making it useful in clinical settings and for personal hygiene.
Oral Hygiene. Often found in mouthwashes, it helps prevent tooth decay and reduce bacterial plaque and halitosis.
Use in Surgery and Medicine. Used for skin cleansing before surgical procedures and for hand disinfection of healthcare personnel.
Safety
This recent study by researchers at the University of São Paulo, Brazil warns of the effects that may affect blood pressure and others caused by chlorhexidine-containing antiseptic mouthwashes versus L-arginine supplementation (5).
For more information: Chlorhexidine studies
| Appearance | White powder |
| Boiling Point | 591.7±60.0°C at 760 mmHg |
| Melting Point | 134-136°C |
| Flash Point | 311.6±32.9°C |
| Density | 1.4±0.1 g/cm3 |
| Vapor Pressure | 0.0±1.7 mmHg at 25°C |
| Refraction Index | 1.659 |
| Water Solubility | 0.08 g/100 mL (20 ºC) |
| PSA | 167.58000 |
| LogP | 4.58 |
| Storage | 2-8°C |
| Safety |  |
 |  |
 |  |
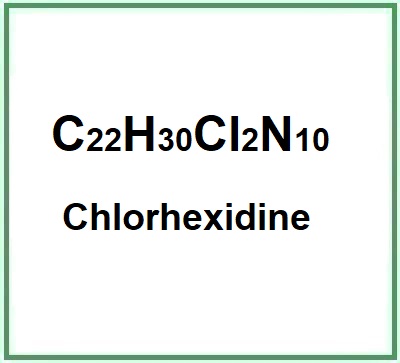
- Molecular Formula C22H30Cl2N10
- Linear Formula [-(CH2)3NHC(=NH)NHC(=NH)NHC6H4Cl]2
- Molecular Weight 505.4
- Massa esatta 504.203186
- CAS 55-56-1
- UNII R4KO0DY52L
- EC Number 200-238-7 223-026-6 200-302-4
- DSSTox Substance ID DTXSID7032345 DTXSID2033314 DTXSID7047144
- IUPAC (1E)-2-[6-[[amino-[(E)-[amino-(4-chloroanilino)methylidene]amino]methylidene]amino]hexyl]-1-[amino-(4-chloroanilino)methylidene]guanidine
- InChI=1S/C22H30Cl2N10/c23-15-5-9-17(10-6-15)31-21(27)33-19(25)29-13-3-1-2-4-14-30-20(26)34-22(28)32-18-11-7-16(24)8-12-18/h5-12H,1-4,13-14H2,(H5,25,27,29,31,33)(H5,26,28,30,32,34)
- InChl Key GHXZTYHSJHQHIJ-UHFFFAOYSA-N
- SMILES C1=CC(=CC=C1NC(=NC(=NCCCCCCN=C(N)N=C(N)NC2=CC=C(C=C2)Cl)N)N)Cl
- MDL number MFCD00009673
- PubChem Substance ID 24857031
- ChEBI 3614
- NCI C364
- RXCUI 2358
- Beilstein 2826432
- RTECS 504.203186
- NACRES NA.22
Synonyms
- 1,1′-Hexamethylenebis[5-(4-chlorophenyl)biguanide]
- Chlorhexidine dihydrochloride
- Chlorhexidine Hydrochloride
References__________________________________________________
(1) Calderini A, Pantaleo G, Rossi A, Gazzolo D, Polizzi E. Adjunctive effect of chlorhexidine antiseptics in mechanical periodontal treatment: first results of a preliminary case series. Int J Dent Hyg. 2013 Aug;11(3):180-5. doi: 10.1111/idh.12009.
(2) Özdemir S, Türk G. The effect of different concentrations of chlorhexidine on microbial colonization: A double-blinded randomized controlled study. Int J Nurs Pract. 2022 Apr;28(2):e13025. doi: 10.1111/ijn.13025.
(3) Rathod AK, Poojari CS, Manna M. Is Lipid Specificity Key to the Potential Antiviral Activity of Mouthwash Reagent Chlorhexidine against SARS-CoV-2? Membranes (Basel). 2022 Jun 14;12(6):616. doi: 10.3390/membranes12060616.
(4) Golac-Guzina, N., Novaković, Z., Sarajlić, Z., Šukalo, A., Džananović, J., Glamočlija, U., Kapo, B., Čordalija, V. and Mehić, M., 2019. Comparative study of the efficacy of the lysozyme, benzydamine and chlorhexidine oral spray in the treatment of acute tonsillopharyngitis-results of a pilot study. Acta Medica Academica, 48(2), pp.140-146.
Abstract. Objective. Lysozyme is a natural antimicrobial and immunomodullatory enzyme, which is produced as a host response to infectious agents. The objective of this study was to compare the efficacy and safety of lysozyme-based versus benzydamine and chlorhexidine-based oral spray in patients with an acute tonsillopharyngitis associaated with a common cold. Patients and Methods. A prospective two-arm pilot study (lysozyme/cetylpyridinium/lidocaine spray versus: benzydamine spray—arm 1; chlorhexidine/lidocaine spray—arm 2) was conducted in the primary health care unit. Efficacy was evaluated by the patient’s self-assessment of pain, difficulty in swallowing and the throat swelling, by using the visual analog scale (VAS) at baseline and three follow-up visits. Safety was evaluated by the assessment of the frequency and severity of adverse effects. Results. Lysozyme-based spray reduced pain faster than benzydamine-based spray and slower than chlorhexidine-based spray. Lysozyme-based and chlorhexidine-based sprays similarly reduced difficulty in swallowing, but were faster than benzydamine-based spray. Similar effects on the reduction of throat swelling were seen in all treated groups. All tested products showed proper safety and were well tolerated, with no serious adverse events reported. Conclusions. The lysozyme-based oral spray was shown to be effective and safe in the reduction of pain, difficulty in swallowing and throat swelling in patients with acute tonsillopharyngitis associated with a common cold. Lysozyme-based oral spray (containing natural compound with advantages of influencing immune system and preventing recurrences) had similar activity to benzydamine and chlorhexidine-based oral antiseptic sprays.
(5) Batista RIM, Nogueira RC, Ferreira GC, Oliveira-Paula GH, Damacena-Angelis C, Pinheiro LC, Tanus-Santos JE. Antiseptic mouthwash inhibits antihypertensive and vascular protective effects of L-arginine. Eur J Pharmacol. 2021 Sep 15;907:174314. doi: 10.1016/j.ejphar.2021.174314.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2022-10-10 08:46:23 | Chemical Risk: |