![]() Borax
Borax
Rating : 5.5
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Antibacterial (1)Cons:
Avoid excessive amounts (1)10 pts from Street82
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Borax studies" about Borax Review Consensus 10 by Street82 (2970 pt) | 2023-Jul-18 18:11 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Ciriza J, Rodríguez-Romano A, Nogueroles I, Gallego-Ferrer G, Cabezuelo RM, Pedraz JL, Rico P. Borax-loaded injectable alginate hydrogels promote muscle regeneration in vivo after an injury. Mater Sci Eng C Mater Biol Appl. 2021 Apr;123:112003. doi: 10.1016/j.msec.2021.112003.
Abstract. This work proposes a different and simple approach to potentiate muscle regeneration exploiting the interplay between specific cell membrane receptors. The simultaneous stimulation of borate transporter, NaBC1 (encoded by SLC4A11gene), and fibronectin-binding integrins induced higher number and size of focal adhesions, major cell spreading and actin stress fibers, strengthening myoblast attachment and providing an enhanced response in terms of myotube fusion and maturation.
Hussain SA, Abood SJ, Gorial FI. The adjuvant use of calcium fructoborate and borax with etanercept in patients with rheumatoid arthritis: Pilot study. J Intercult Ethnopharmacol. 2016 Dec 8;6(1):58-64. doi: 10.5455/jice.20161204021549.
Abstract. The use of boron, as adjuvant with etanercept, has potentiated therapeutic outcomes in rheumatoid arthritis patients, and may be a new strategy to improve treatment, and avoid the problems associated with biologics utilized in rheumatoid arthritis treatment.
Hadrup N, Frederiksen M, Sharma AK. Toxicity of boric acid, borax and other boron containing compounds: A review. Regul Toxicol Pharmacol. 2021 Apr;121:104873. doi: 10.1016/j.yrtph.2021.104873.
Abstract. We assessed the toxicity of boric acid, borax and other forms of boron, after inhalation, dermal and oral exposure.
Tanpichai S, Phoothong F, Boonmahitthisud A. Superabsorbent cellulose-based hydrogels cross-liked with borax. Sci Rep. 2022 May 26;12(1):8920. doi: 10.1038/s41598-022-12688-2.
Abstract. Cellulose, the most abundant biopolymer on Earth, has been widely attracted owing to availability, intoxicity, and biodegradability. Environmentally friendly hydrogels were successfully prepared from water hyacinth-extracted cellulose using a dissolution approach with sodium hydroxide and urea, and sodium tetraborate decahydrate (borax) was used to generate cross-linking between hydroxyl groups of cellulose chains. The incorporation of borax could provide the superabsorbent feature into the cellulose hydrogels.
Ahmed M, Hussein IA, Onawole AT, Saad MA. Development of a New Borax-Based Formulation for the Removal of Pyrite Scales. ACS Omega. 2020 Jun 12;5(24):14308-14315. doi: 10.1021/acsomega.0c00556.
Abstract. In the oil and gas industry, pyrite forms one of the most hardened scales in reservoirs, which hinders the flow of fluids. Consequently, this leads to blockage of the downhole tubular, formation damage, and complete shutdown of production and operational processes. Herein, a new green formulation based on borax (K2B4O7) is proposed for pyrite scale removal. The temperature effect, disk rotational speed, and borax concentration have been investigated using a rotating disk apparatus. Also, XPS and SEM-EDX analyses were conducted on the pyrite disk surface before and after the treatment with the green formulation.
Zhai X, Gao S, Xiang Y, Wang A, Li Z, Cui B, Wang W. Cationized high amylose maize starch films reinforced with borax cross-linked nanocellulose. Int J Biol Macromol. 2021 Dec 15;193(Pt B):1421-1429. doi: 10.1016/j.ijbiomac.2021.10.206.
Abstract. In this study, a novel strategy for modifying nanocellulose (NC) by borax cross-linking was developed, and the obtained borax modified nanocellulose (BNC) was incorporated into cationized high amylose maize starch (CS) films to evaluate the applicability.
Chen X, Ji N, Li F, Qin Y, Wang Y, Xiong L, Sun Q. Dual Cross-Linked Starch-Borax Double Network Hydrogels with Tough and Self-Healing Properties. Foods. 2022 Apr 30;11(9):1315. doi: 10.3390/foods11091315.
Abstract. Herein, we have fabricated starch-borax double cross-linked network (DC) hydrogels with tough and self-healing properties using a one-pot method. The addition of borax significantly increased the storage modulus and loss modulus of these starch-borax DC hydrogels.
Tantiwatcharothai S, Prachayawarakorn J. Property improvement of antibacterial wound dressing from basil seed (O. basilicum L.) mucilage- ZnO nanocomposite by borax crosslinking. Carbohydr Polym. 2020 Jan 1;227:115360. doi: 10.1016/j.carbpol.2019.115360.
Abstract. Some applications, in particular, wound dressings, require significant water holding capability: hydrogels formed from Basil seed mucilage (BSM) are non-toxic natural substances and exhibit the needed water holding capacity. However, the sponges have low dimensional stability and easily degrade in aqueous media. We overcame this drawback by crosslinking with borax.
Ince S, Kucukkurt I, Cigerci IH, Fatih Fidan A, Eryavuz A. The effects of dietary boric acid and borax supplementation on lipid peroxidation, antioxidant activity, and DNA damage in rats. J Trace Elem Med Biol. 2010 Jul;24(3):161-4. doi: 10.1016/j.jtemb.2010.01.003.
Abstract. The aims of this study were to clarify the effects of high dietary supplementation with boric acid and borax, called boron (B) compounds, on lipid peroxidation (LPO), antioxidant activity, some vitamin levels, and DNA damage in rats.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Borax Review Consensus 10 by Street82 (2970 pt) | 2023-Jul-18 18:32 |
| Read the full Tiiip | (Send your comment) |
Borax or Sodium Tetraborate or Sodium borate, is a mineral and chemical compound in the refined form of natural sodium borate. It is composed of boron trioxide, sodium oxide and water.
Description of the raw materials:
- Boracite - A boron mineral from which borax is extracted. Boracite is found in evaporite deposits in various parts of the world.
- Water - Water is used in the extraction and purification process of borax.
Borax can be found in nature in the form of minerals such as borax. Therefore, its natural synthesis involves the process of extracting and purifying borax-containing minerals.
- Extraction of borax mineral. The borax-containing mineral is extracted from the mine or natural sources.
- Refining. The borax mineral undergoes refining processes to remove any impurities and obtain a pure Borax product.
- Purification. The extracted Borax is purified through washing and filtration processes to remove any residual impurities.
- Drying. The purified Borax is dried to remove residual moisture and obtain a solid product.
- Crushing and grinding. The solid product is crushed and ground to obtain the desired form, such as powder or crystals.
Summary of the industrial chemical synthesis process:
- Preparation of reagents. The reagents needed for the synthesis of Borax, such as boric acid and sodium carbonate, are collected and prepared.
- Chemical reaction. Boric acid and sodium carbonate react with each other in a reactive system, such as a chemical reactor, to form sodium borate, which is Borax.
- Crystallization. The solution containing sodium borate is cooled and subjected to crystallization processes to precipitate Borax crystals.
- Filtration and washing. The Borax crystals are separated from the solution through filtration and washing processes to remove any impurities or residues.
- Drying. The Borax crystals are dried to remove moisture and obtain a solid product.
- Crushing and grinding. The solid product is crushed and ground to obtain the desired form of Borax, such as powder or crystals.
- Packaging of Borax in appropriate containers for distribution and use.
It appears as a white, crystalline, alkaline granular powder that melts in glass at 878°C. Easily soluble in water and glycerine, it is efflorescent when exposed to dry air.

What it is used for and where
Medical
The addition of borax, for tissue engineering applications on mesenchymal stem cells, induces osteogenesis by stimulating growth factor receptors and fibronectin-binding integrins (1).
The use of borax or boric acid buffers in ophthalmology is also approved by the Medicines and Healthcare products Regulatory Agency for paediatric use in children under 2 years of age (2). It acts as a mild antiseptic and cleaning agent.
In biomedicine, borax hydrogels, together with locust bean gum and gellan gum, have demonstrated good mechanical properties with excellent self-healing and thermo-processability properties (3).
Present in tooth-whitening formulas.
Food
A food additive labelled E285 on the European food additive list as a preservative, but banned in the US, Thailand, China and the current trend is to reduce or eliminate its use. In 2013, the European Food Safety Authority (EFSA) stated that boric acid and its salts are toxicologically acceptable as preservatives in caviar as the intake of this expensive fish product does not occur on a regular basis and it is considered unlikely that the boron dose of 0.16 mg per kg body weight/day (ADI) would be exceeded (4).
Safety
Borax is toxic to humans and can induce reproductive and developmental toxicity, neurotoxicity and nephrotoxicity.
Borax concentrations above 52 mM (20 mg mL-1) are toxic to cells, while borax concentrations above 15.7 mM (6 mg mL-1) inhibit the formation of myotubes (5), a type of cell that develops into muscle fibres.
Jewellery
Melting metals such as gold and silver and for their cleaning as borax removes oxides on the metal surface and, when mixed with water, improves melting point uniformity. It softens tungsten in pre-soldering with zinc and improves solderability.
Replaces mercury in gold mining in small-scale mines with less toxic characteristics.
Cosmetics
It is a restricted ingredient as II/1396 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Substance or ingredient reported: Boric acid, Borates and Tetraborates, Sodium Perborate and Perboric Acid
- Buffering agent. It is an iingredient that can bring an alkaline or acid solution to a certain pH level and prevent it from changing, in practice a pH stabiliser that can effectively resist instability and pH change.
Agriculture
It is considered a dynamic covalent cross-linking agent as a water retention agent.
Insecticides
Useful as an ant and insect repellent.
Biochemistry
Used as a buffer in biochemical and chemical laboratories and nucleic acid buffer most commonly used in DNA gel electrophoresis, as TBE or BBS, saline buffered borate gel, SB, gel without chemical reactions or solvents, SB or BBS (saline buffered borate) in coating procedures. Borate buffers (usually pH 8) are also used as the preferred equilibrium solution in DMP-based cross-linking reactions.
Other uses
Chemical cross-linking agent. Flame retardant in insulation products in fireproofing paints, textile stain removal, porcelain manufacture.
Glass. Production of high quality glass. Raw material for boron oxide in the manufacture of thermally insulated glass wool, heat-resistant glass, borosilicate glass, glass fibre weaving, optical glasses, sealing glass.
Metals. When welding iron and steel, mixed borax and ammonium chloride are used to lower the melting point of unwanted iron oxide. In metal drawing it functions as a lubricant carrier.
Construction. Slackener in cement and concrete.
Chemical industry. In paraffin production it is the pH value buffering agent in the water system and emulsifier.
Water treatment.
In compounds containing boron, borax is the basic raw material.
Fertiliser in agriculture.
Also: detergent, rust binder, fire retardant, emulsifier, stabiliser etc.
For more information:
Typical commercial product characteristics Sodium tetraborate decahydrate
| Appearance | White granular powder |
| Boiling Point | 320°C |
| Melting Point | 75°C |
| Density | 1.73 g/mL at 25°C(lit.) |
| PSA | 184.57000 |
| Water Solubility | 60 g/L (20 ºC) |
| Boron monoxide B2O | ≥ 36.47% |
| Sulfur dioxide SO2 | ≤ 0.1% |
| Chloride Cl | ≤ 0.05% |
| Iron Fe | ≤ 0.001% |
| Sodium oxide Na2O | ≥16% |
| Boron trioxide B2O3 | ≥ 35% |
| Insoluble in water | 0.04% Max |
| Safety |  |
 |  |
 |  |
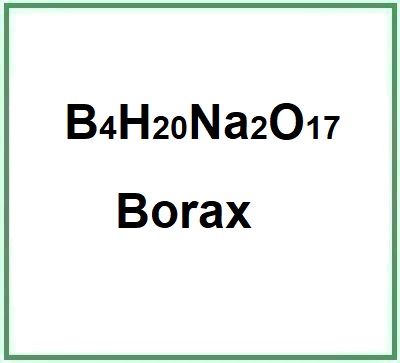
- Molecular Formula B4H20Na2O17 B4O7Na2. 10H2O
- Linear Formula Na2B4O7 · 10H2O
- Molecular Weight 381.4
- Exact Mass 382.086823
- CAS 1303-96-4
- UNII 91MBZ8H3QO
- EC Number 603-411-9 215-540-4
- DSSTox Substance ID DTXSID701014356 DTXSID2034384
- IUPAC disodium;3,7-dioxido-2,4,6,8,9-pentaoxa-1,3,5,7-tetraborabicyclo[3.3.1]nonane;decahydrate
- InChI=1S/B4O7.2Na.10H2O/c5-1-7-3-9-2(6)10-4(8-1)11-3;;;;;;;;;;;;/h;;;10*1H2/q-2;2*+1;;;;;;;;;;
- InChl Key CDMADVZSLOHIFP-UHFFFAOYSA-N
- SMILES B1(OB2OB(OB(O1)O2)[O-])[O-].O.O.O.O.O.O.O.O.O.O.[Na+].[Na+]
- MDL number MFCD00149193
- PubChem Substance ID 329824630
- RXCUI 36680
- ICSC 0567
- NACRES NA.21
- RTECS VZ2275000
Synonyms
- Sodium tetraborate decahydrate
- Borax decahydrate
- Sodium borate decahydrate
- Sodium pyroborate decahydrate
References_______________________________________________________
(1) Rico P, Rodrigo-Navarro A, Sánchez Pérez L, Salmeron-Sanchez M. Borax induces osteogenesis by stimulating NaBC1 transporter via activation of BMP pathway. Commun Biol. 2020 Nov 27;3(1):717. doi: 10.1038/s42003-020-01449-4.
(2) Chloramphenicol eye drops: ok to use in children younger than 2 years. Drug Ther Bull. 2021 Sep;59(9):133. doi: 10.1136/dtb.2021.000045.
(3) Lv Y, Pan Z, Song C, Chen Y, Qian X. Locust bean gum/gellan gum double-network hydrogels with superior self-healing and pH-driven shape-memory properties. Soft Matter. 2019 Aug 14;15(30):6171-6179. doi: 10.1039/c9sm00861f.
(4) EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). (2013). Scientific Opinion on the re‐evaluation of boric acid (E 284) and sodium tetraborate (borax)(E 285) as food additives. EFSA Journal, 11(10), 3407.
(5) Ciriza J, Rodríguez-Romano A, Nogueroles I, Gallego-Ferrer G, Cabezuelo RM, Pedraz JL, Rico P. Borax-loaded injectable alginate hydrogels promote muscle regeneration in vivo after an injury. Mater Sci Eng C Mater Biol Appl. 2021 Apr;123:112003. doi: 10.1016/j.msec.2021.112003.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2022-07-28 17:39:55 | Chemical Risk: |