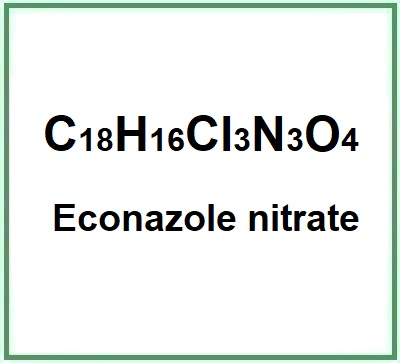
Econazole nitrate
Pros:
Antifungal (1) Antibacterial (1)Cons:
Take only under medical supervision (1)10 pts from Whiz35
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Econazole nitrate studies" about Econazole nitrate Review Consensus 10 by Whiz35 (11840 pt) | 2022-Aug-18 17:23 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Jansook P, Prajapati M, Pruksakorn P, Loftsson T. Antifungal activity of econazole nitrate/cyclodextrin complex: Effect of pH and formation of complex aggregates. Int J Pharm. 2020 Jan 25;574:118896. doi: 10.1016/j.ijpharm.2019.118896.
Abstract.Econazole nitrate (ECN) is a weakly basic drug with very low aqueous solubility that hampers its permeation through biological membranes and results in low ECN bioavailability. Formation of drug/cyclodextrin (drug/CD) inclusion complexes is a formulation technology that can be applied to enhance drug solubility in aqueous media. The aim of this study was to determine the effect of CD complexation and pH adjustments on the ECN solubility. ...
Elewski BE, Vlahovic TC. Econazole nitrate foam 1% for the treatment of tinea pedis: results from two double-blind, vehicle-controlled, phase 3 clinical trials. J Drugs Dermatol. 2014 Jul;13(7):803-8.
Abstract. Econazole nitrate is a broad-spectrum topical antifungal with activity against a variety of dermatophytes and yeasts. A new topical dosage form, econazole nitrate topical foam 1%, utilizing patented Proderm Technology® has been developed for treatment of interdigital tinea pedis....To evaluate econazole nitrate foam 1% versus foam vehicle for treatment of interdigital tinea pedis....
Crawford F. Athlete's foot. BMJ Clin Evid. 2009 Jul 20;2009:1712.
Abstract. ...We conducted a systematic review and aimed to answer the following clinical question: What are the effects of topical treatments for athlete's foot? We searched: Medline, Embase, The Cochrane Library, and other important databases up to July 2008 (Clinical Evidence reviews are updated periodically; please check our website for the most up-to-date version of this review). We included harms alerts from relevant organisations such as the US Food and Drug Administration (FDA) and the UK Medicines and Healthcare products Regulatory Agency (MHRA)....
Srivastava S, Mahor A, Singh G, Bansal K, Singh PP, Gupta R, Dutt R, Alanazi AM, Khan AA, Kesharwani P. Formulation Development, In Vitro and In Vivo Evaluation of Topical Hydrogel Formulation of Econazole Nitrate-Loaded β-Cyclodextrin Nanosponges. J Pharm Sci. 2021 Nov;110(11):3702-3714. doi: 10.1016/j.xphs.2021.07.008.
Abstract. Econazole nitrate, an antifungal drug used in the handling of skin ailments, is commercially not efficient as these ailments typically require a more elevated concentration of the drug to offer an effective pharmacological retort. Like so, it is proposed to assess the effectiveness of the topical hydrogel of econazole-loaded nanosponge in the management of skin ailment(s). ...
Albertini B, Passerini N, Di Sabatino M, Vitali B, Brigidi P, Rodriguez L. Polymer-lipid based mucoadhesive microspheres prepared by spray-congealing for the vaginal delivery of econazole nitrate. Eur J Pharm Sci. 2009 Mar 2;36(4-5):591-601. doi: 10.1016/j.ejps.2008.12.009.
Abstract. This research aimed to evaluate a new approach for the preparation of mucoadhesive microparticles and to design an innovative vaginal delivery systems for econazole nitrate (ECN) able to enhance the drug antifungal activity. ...
Kokjohn K, Bradley M, Griffiths B, Ghannoum M. Evaluation of in vitro activity of ciclopirox olamine, butenafine HCl and econazole nitrate against dermatophytes, yeasts and bacteria. Int J Dermatol. 2003 Sep;42 Suppl 1:11-7. doi: 10.1046/j.1365-4362.42.s1.4.x.
Abstract. In many instances, a cutaneous fungal infection may exist concomitantly with bacterial involvement. In this study we compared the in vitro activity of three antifungal agents against the dermatophytes, yeasts and bacteria recovered most commonly from cutaneous mycoses and bacterial infections....
Gajra B, Pandya SS, Singh S, Rabari HA. Mucoadhesive hydrogel films of econazole nitrate: formulation and optimization using factorial design. J Drug Deliv. 2014;2014:305863. doi: 10.1155/2014/305863.
Abstract. The mucoadhesive hydrogel film was prepared and optimized for the purpose of local drug delivery to oral cavity for the treatment of oral Candidiasis. The mucoadhesive hydrogel film was prepared with the poly(vinyl alcohol) by freeze/thaw crosslinking technique. 3(2) full factorial design was employed to optimize the formulation. Number of freeze/thaw cycles (4, 6, and 8 cycles) and the concentration of the poly(vinyl alcohol) (10, 15, and 20%) were used as the independent variables whereas time required for 50% drug release, cumulative percent of drug release at 8th hour, and "k" of zero order equation were used as the dependent variables. The films were evaluated for mucoadhesive strength, in vitro residence time, swelling study, in vitro drug release, and effectiveness against Candida albicans. The concentration of poly(vinyl alcohol) and the number of freeze/thaw cycles both decrease the drug release rate. Mucoadhesive hydrogel film with 15% poly(vinyl alcohol) and 7 freeze/thaw cycles was optimized. The optimized batch exhibited the sustained release of drug and the antifungal studies revealed that the drug released from the film could inhibit the growth of Candida albicans for 12 hours.
Ambrogi V, Perioli L, Pagano C, Marmottini F, Moretti M, Mizzi F, Rossi C. Econazole nitrate-loaded MCM-41 for an antifungal topical powder formulation. J Pharm Sci. 2010 Nov;99(11):4738-45. doi: 10.1002/jps.22183.
Abstract. The aim of this article was to prepare a topical powder for the treatment of fungal infections, such as Candida intertrigo and tinea pedis. ...
Verma S, Utreja P. Transethosomes of Econazole Nitrate for Transdermal Delivery: Development, In-vitro Characterization, and Ex-vivo Assessment. Pharm Nanotechnol. 2018;6(3):171-179. doi: 10.2174/2211738506666180813122102.
Abstract. The main objective of the present research work was to develop and characterize (in-vitro and ex-vivo) econazole nitrate loaded transethosomes and their comparison with marketed cream of econazole nitrate [Ecoderm, Brown and Burk Pharmaceutical (Pvt.) Ltd., Bengaluru, India] for effective transdermal delivery.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Econazole nitrate Review Consensus 10 by Whiz35 (11840 pt) | 2023-Sep-11 10:29 |
| Read the full Tiiip | (Send your comment) |
Econazole nitrate is a chemical compound, an econazole derivative that was, synthesised in 1969 and placed on the commercial market in the 1970s.
It appears as an odourless whitish crystalline powder with low solubility in water. Stable, incompatible with strong oxidising agents.

What it is used for and where
Medical
Take only under medical supervision
Econazole nitrate is a topical antifungal and bactericidal agent with an antibacterial action that inhibits the biosynthesis of sterols such as ergosterol that regulate fungal cell wall permeability. It also interrupts the oxidative and peroxidative process of fungal enzymes that create cell necrosis.
An antifungal agent that has shown a broad spectrum of activity against Candida albicans, Aspergillus and Staphylococcus, commonly used to treat general fungal infections, fungal infections of the skin, such as
- dermatophytic infection of athlete's foot (Tinea pedis) localised in the interdigital spaces of the toes, caused by the fungi Trichophyton rubrum, Epidermophyton floccosum, Trichophyton mentagrophytes
- tinea versicolor or pityriasis versicolor (mycosis) caused by the mycete Malassezia furfur, ringworm,
- itchy socks
and other infections caused by certain Gram-positive bacteria, filamentous fungi (Rhizopus spp), yeasts and moulds. In the spray dosage form, econazole is added in 1%, dissolved in ethanol (25%) and isopropyl alcohol (5%), but excipients may be different and in lower percentages. For example, propylene glycol is added in concentrations ranging from 5% to 10% (1).
This study analysed the properties of Econazole in laboratory animals, found that Econazole significantly suppressed the growth of lung cancer cells and concluded that Econazole could be used in the treatment of lung cancer alone or in combination with cisplatin (2).
Another interesting application of Econazole nitrate could prove to be ultrasound-assisted transdermal administration as a new therapy for Raynaud's phenomenon, which is an abnormal spasm of blood vessels that causes tingling, cyanosis and most frequently affects the hands (3).
Onychomycosis is a common nail disease that can increase with age and predisposing factors are abnormalities in foot development, impaired blood flow. the main aetiological agent is the fungus Trichophyton rubrum, while the second pathogen is Тrichophyton mentagrophytes var. interdigitale. Econazole nitrate, as a systemic antimycotic, is able to eradicate both pathogenic fungi by enhancing the antifungal effect and reducing the likelihood of secondary bacterial infection.
Like all drugs it can cause side effects. Always ask the physician.
Price
- 5 g €66.00
- 25 g €246.00
- 100 g €644.00
| Appearance | Off white powder |
| Melting Point | 158 - 166°C |
| PSA | 93.10000 |
| LogP | 5.97690 |
| Solubility | 1 mM 2.2487 mL 11.2435 mL 22.4871 mL 5 mM 0.4497 mL 2.2487 mL 4.4974 mL 10 mM 0.2249 mL 1.1244 mL 2.2487 mL |
| Storage | Powder -20°C 3 years or 4°C 2 years As solvent -80°C 6 months or -20°C 1 month |
| Safety |  |
 |  |
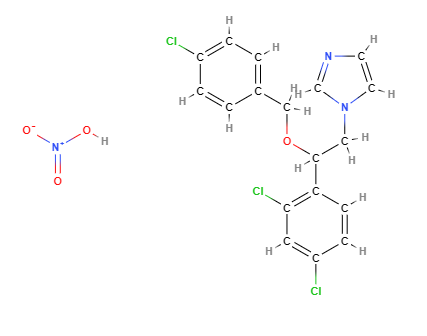 |  |
- Molecular Formula C18H16Cl3N3O4 o C18H15Cl3N2O.HNO3
- Molecular Weight 444.7
- Exact Mass 443.02100
- CAS 24169-02-6 68797-31-9
- UNII H438WYN10E
- EC Number 246-053-5 272-295-6
- DSSTox Substance ID DTXSID6025226
- IUPAC 1-[2-[(4-chlorophenyl)methoxy]-2-(2,4-dichlorophenyl)ethyl]imidazole;nitric acid
- InChI=1S/C18H15Cl3N2O.HNO3/c19-14-3-1-13(2-4-14)11-24-18(10-23-8-7-22-12-23)16-6-5-15(20)9-17(16)21;2-1(3)4/h1-9,12,18H,10-11H2;(H,2,3,4)
- InChl Key DDXORDQKGIZAME-UHFFFAOYSA-N
- SMILES C1=CC(=CC=C1COC(CN2C=CN=C2)C3=C(C=C(C=C3)Cl)Cl)Cl.[N+](=O)(O)[O-]
- MDL number MFCD00058160
- PubChem Substance ID 24894567
- CHEMBL1201049
- SMR000058732
- HS code 2933290090
- NSC 757882 243115
- RTECS NI4450000
- NACRES NA.85
Synonyms
- 1-(2-[(4-Chlorophenyl)methoxy]-2-[2,4-dichlorophenyl]ethyl)-1H-imidazole
- 1-{2,4-Dichloro-β-[(p-chlorobenzyl)oxy}phenethyl]imidazole mononitrate
References_______________________________________________________________________
(1) Kapadia MM, Solanki ST, Parmar V, Thosar MM, Pancholi SS. Preliminary investigation tests of novel antifungal topical aerosol. J Pharm Bioallied Sci. 2012 Mar;4(Suppl 1):S74-6. doi: 10.4103/0975-7406.94145.
(2) Dong C, Yang R, Li H, Ke K, Luo C, Yang F, Shi XN, Zhu Y, Liu X, Wong MH, Lin G, Wang X, Leung KS, Kung HF, Chen C, Lin MC. Econazole nitrate inhibits PI3K activity and promotes apoptosis in lung cancer cells. Sci Rep. 2017 Dec 21;7(1):17987. doi: 10.1038/s41598-017-18178-0.
(3) Daftardar S, Bahl D, Boddu SHS, Altorok N, Kahaleh B. Ultrasound-mediated topical delivery of econazole nitrate with potential for treating Raynaud's phenomenon. Int J Pharm. 2020 Apr 30;580:119229. doi: 10.1016/j.ijpharm.2020.119229.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances:
Last update: 2022-08-18 15:20:13 | Chemical Risk: |