![]() Polysorbate-20
Polysorbate-20
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Cons:
To be taken in controlled quantity (1)10 pts from Whiz35
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about Polysorbate-20 Review Consensus 10 by Whiz35 (11840 pt) | 2024-Sep-21 19:03 |
| Read the full Tiiip | (Send your comment) |
Polysorbate 20 is a non-ionic surfactant and emulsifier derived from sorbitol and fatty acids, specifically lauric acid. It is widely used in cosmetics and personal care products for its ability to stabilize emulsions and enhance the texture of formulations. Polysorbate 20 is valued for its mildness, making it suitable for a variety of skin and hair care applications.
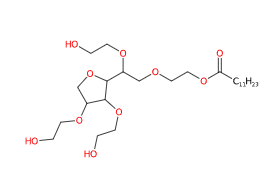
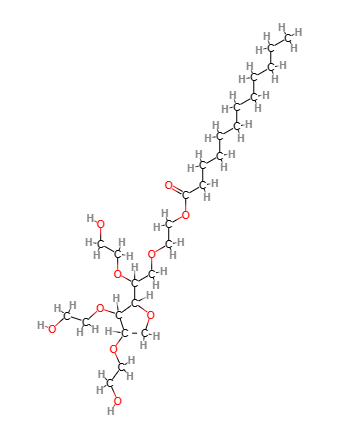
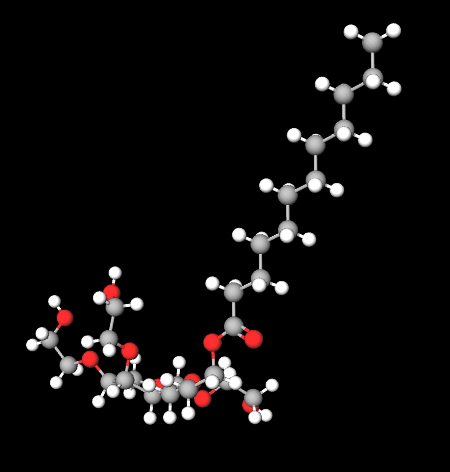
Chemical Composition and Structure
The chemical composition of Polysorbate 20 includes:
- Sorbitan Monolaurate: The base structure is formed from sorbitol and lauric acid, which is then reacted with ethylene oxide.
- Ethylene Oxide Units: The addition of ethylene oxide creates a hydrophilic polymer chain, contributing to its surfactant properties.
Structurally, Polysorbate 20 features a hydrophilic head (from the ethylene oxide units) and a hydrophobic tail (from lauric acid), allowing it to effectively stabilize oil-in-water emulsions.
Physical Properties
Appearance: Typically a clear to slightly hazy liquid.
Solubility: Soluble in water and various organic solvents; well-compatible with many cosmetic ingredients.
pH: Generally neutral to slightly acidic, depending on the formulation.
Odor: Mild or odorless.
Stability: Stable under normal storage conditions; should be protected from extreme temperatures.
Production Process
Synthesis: Polysorbate 20 is synthesized by the reaction of sorbitan monolaurate with ethylene oxide under controlled conditions to achieve the desired degree of ethoxylation.
Purification: The product is purified to remove any unreacted materials and impurities, ensuring high quality.
Formulation: Purified Polysorbate 20 is incorporated into various cosmetic products to enhance their performance.
Appears as a white powder or as a stable yellowish viscous liquid. Incompatible with strong oxidising agents. Soluble in water, ethanol, methanol, carbinol, isopropanol, isopropyl alcohol and other solvents, insoluble in mineral oil.


What it is used for and where
Chemical auxiliary agent, chemical intermediate, hydrophilic and non-ionic surfactant. Generally regarded as a fatty acid ethoxylated sorbitan ester.
Medical and pharmaceuticals
Widely used in biopharmaceutical protein formulations as a stabilising excipient in emulsions and suspensions and to minimise their binding to surfaces during storage and purification (1). It is an oil-in-water emulsifier, and is used as a solubiliser, diffusing agent, antistatic agent, and lubricant in biopharmaceutical products. Polysorbate 20 among non-ionic emulsifiers selected on the physico-chemical characteristics of astaxanthin (a potent antioxidant) nanodispersions produced by an emulsification/evaporation technique, demonstrated the lowest astaxanthin loss rate (2). This shows how important the choice of emulsifier type is in cosmetic and pharmaceutical formulations.
An interesting behaviour of Polysorbate 20 was observed in this study, which also analysed another non-ionic surfactant, Poloxamer 188. The structural changes of the proteins during freezing/thawing were found to be different (3).
Finally, with regard to safety, Polysorbate 20 is considered safe as an excipient at a level similar to that of human serum albumin even when administered intramuscularly (4). It must be remembered, however, that excessive doses of polysorbate 20 may cause mild to moderate irritation and, if administered orally, may cause even more serious effects (5).
Cosmetics
Amphiphilic non-ionic surfactant with dispersing and emulsifying activity in oil-in-water solutions to improve the solubility and stability of cosmetic formulations.
Surfactant - Cleansing agent. Cosmetic products used to cleanse the skin utilise the surface-active action that produces a lowering of the surface tension of the stratum corneum, facilitating the removal of dirt and impurities.
Surfactant - Emulsifying agent. Emulsions are thermodynamically unstable and are used to soothe or soften the skin and emulsify, so they need a specific, stabilising ingredient. This ingredient forms a film, lowers the surface tension and makes two immiscible liquids miscible. A very important factor affecting the stability of the emulsion is the amount of the emulsifying agent. Emulsifiers have the property of reducing the oil/water or water/oil interfacial tension, improving the stability of the emulsion and also directly influencing the stability, sensory properties and surface tension of sunscreens by modulating the filmometric performance.
Food
Ingredient included in the list of European food additives such as E432 and used as penetrant, solubilizing and emulsifier.
Safety
The Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO)Expert Committee on Food Additives (JECFA)derived an Acceptable Daily Intake (ADI) of 25mg/kg body weight (bw)/day (group ADI for polysorbates 20, 40, 60, 65 and 80) and the Scientific Committee on Food (SCF) derived a group ADI of 10mg/kg bw/day. Small amounts of polyoxyethylene sorbitansare absorbed. Similar toxicokinetics would be expected for all polysorbates based on their similarities in structure and metabolic fate. The acute toxicity is very low. There is no concern regarding genotoxicity, carcinogenicity or developmental toxicity. From a limited number of studies,there is no indication of reproductive toxicity. The Panel recommended that the maximum limits for the impurities of toxic elements (arsenic, lead, cadmium and mercury) in the EC specification for polysorbates (E432–E436) should be revised in order to ascertain that polysorbates (E432–E436) as food additives will not be a significant source of exposure to those toxic elements in food (6).
Other uses
- Wax inhibitor for oil production.
- Antistatic agent, softener, lubricant in the textile industry.
- Solvent for dyes.
- Stabiliser for foamed plastics.
- Lubricant and diffuser in pesticides.
For more information: Polysorbate 20 studies
Typical commercial product characteristics Tween 20
| Appearance | White powder or yellow liquid |
| pH | 6-8 (50g/l, H2O, 20℃) |
| Boiling Point | 695.8±55.0°C at 760 mmHg 100°C |
| Melting Point | 98.9°C |
| Flash Point | 207.1±25.0°C |
| Density | 1.1±0.1 g/cm3 at 20°C |
| Vapor Pressure | 0.0±5.0 mmHg at 25°C <1.4 hPa (20°C) |
| PSA | 133.14000 |
| LogP | 5.37 |
| Refraction Index | 1.502 n20/D 1.468(lit.) |
| Water Solubility | 100 g/L |
| Water | ≤3.0% |
| Specific Gravity | 1.090~1.130 (20/20℃) |
| HLB | 16.7 |
| Acid value | ≤2.0 |
| Saponification value | 40-50 (mg KOH/g) |
| Hydroxyl value | 96-108 (mg KOH/g) |
| Lead | ≤2 (mg/kg) |
| Oxyethylene | 72.0% |
| Storage | 0-6°C |
| Shelf Life | 2 Years |
 |  |
 |  |
Price
25 ml $14.90
250 ml $39.50
1 l $ 75.00
- Molecular Formula C26H50O10
- Molecular Weight 604.813
- CAS 9005-64-5
- UNII
- EC Number 500-018-3
- DSSTox Substance ID DTXSID3031949 DTXSID60922815 DTXSID70897060
- IUPAC 2-[2-[3,4-bis(2-hydroxyethoxy)oxolan-2-yl]-2-(2-hydroxyethoxy)ethoxy]ethyl dodecanoate
- InChI=1S/C26H50O10/c1-2-3-4-5-6-7-8-9-10-11-24(30)34-19-18-31-20-22(32-15-12-27)26-25(35-17-14-29)23(21-36-26)33-16-13-28/h22-23,25-29H,2-21H2,1H3
- InChl Key HMFKFHLTUCJZJO-UHFFFAOYSA-N
- SMILES CCCCCCCCCCCC(=O)OCCOCC(C1C(C(CO1)OCCO)OCCO)OCCO
- MDL number MFCD00165986
- PubChem Substance ID
- NACRES NA.21
- FEMA 2915
- RTECS TR7400000
- HS Code 34021300
Synonyms
- 2-[2-[3,4-bis(2-hydroxyethoxy)oxolan-2-yl]-2-(2-hydroxyethoxy)ethoxy]ethyl dodecanoate
- TW 20
- Tween 20
- Hexofuranoside, 2-hydroxyethyl 2-deoxy-3,5-bis-O-(2-hydroxyethyl)-6-O-[2-[[(9E)-1-oxo-9-octadecen-1-yl]oxy]ethyl]-
- Polyoxyethylene (20) sorbitan monolaurate
- polyoxyethylene sorbitan monolaurate
- hexofuranoside, 2-hydroxyethyl 2-deoxy-3,5-bis-O-(2-hydroxyethyl)-6-O-[2-[[(9E)-1-oxo-9-octadecenyl]oxy]ethyl]-
- 2-[2-[3,4-bis(2-hydroxyethoxy)oxolan-2-yl]-2-(2-hydroxyethoxy)ethoxy]ethyl dodecanoate
References____________________________________________________________________
(1) Ionova Y, Wilson L. Biologic excipients: Importance of clinical awareness of inactive ingredients. PLoS One. 2020 Jun 25;15(6):e0235076. doi: 10.1371/journal.pone.0235076.
Abstract. Due to the complexity and fragility of biological drug products, several challenges exist in their formulation development. Excipients are added to increase product stability, maintain tonicity, and facilitate drug delivery. The potential implications of these additive substances merit clinical consideration. We assessed the safety risk of excipients on the basis of their type and variability through an assessment framework, which quantifies excipient complexity in 230 biological formulations, and identifies excipient-related adverse events through published case reports. A biologic on average contained 4.45 excipients, half of that found in oral medications. The frequency distribution was heavily skewed towards the most commonly occurring excipients: water (40.4%), sodium chloride (38.3%), polysorbate 80 (28.7%), sucrose (24.4%), and mannitol (20.9%), with 44.4% of formulations not listing the concentration of the most commonly occurring inactive ingredients. A literature search revealed only 17 case reports of excipient-related adverse events, suggesting the need for more clarity for clinicians on the safety of chemical additives. These cases included injection site reactions, anaphylaxis, hyperglycemia, and acute renal failure. With the expansion of the biopharmaceutical market, it is important to consider the safety data of biologic excipients, so that therapy can be tailored appropriately for a specific patient.
(2) Anarjan, N., & Tan, C. P. (2013). Effects of selected polysorbate and sucrose ester emulsifiers on the physicochemical properties of astaxanthin nanodispersions. Molecules, 18(1), 768-777.
Abstract. The effects of selected nonionic emulsifiers on the physicochemical characteristics of astaxanthin nanodispersions produced by an emulsification/evaporation technique were studied. The emulsifiers used were polysorbates (Polysorbate 20, Polysorbate 40, Polysorbate 60 and Polysorbate 80) and sucrose esters of fatty acids (sucrose laurate, palmitate, stearate and oleate). The mean particle diameters of the nanodispersions ranged from 70 nm to 150 nm, depending on the emulsifier used. In the prepared nanodispersions, the astaxanthin particle diameter decreased with increasing emulsifier hydrophilicity and decreasing carbon number of the fatty acid in the emulsifier structure. Astaxanthin nanodispersions with the smallest particle diameters were produced with Polysorbate 20 and sucrose laurate among the polysorbates and the sucrose esters, respectively. We also found that the Polysorbate 80- and sucrose oleate-stabilized nanodispersions had the highest astaxanthin losses (i.e., the lowest astaxanthin contents in the final products) among the nanodispersions. This work demonstrated the importance of emulsifier type in determining the physicochemical characteristics of astaxanthin nano-dispersions.
(3) Yuan X, Krueger S, Shalaev E. Protein-surfactant and protein-protein interactions during freeze and thaw: a small-angle neutron scattering study of lysozyme solutions with polysorbate and poloxamer. J Pharm Sci. 2022 Aug 19:S0022-3549(22)00351-3. doi: 10.1016/j.xphs.2022.08.017.
(4) Kim J, Kwak S, Park MS, Rhee CH, Yang GH, Lee J, Son WC, Kang WH. Safety verification for polysorbate 20, pharmaceutical excipient for intramuscular administration, in Sprague-Dawley rats and New Zealand White rabbits. PLoS One. 2021 Aug 27;16(8):e0256869. doi: 10.1371/journal.pone.0256869.
Abstract. Human serum albumin (HSA) has been widely used as a pharmaceutical excipient in Botulinum toxin serotype A (BoNT/A) products that are indicated for use in therapeutics and cosmetics. However, HSA as a human-derived material has some concerns, such as the potential risk of transmission of infectious agents, an insufficient supply, and difficulty in maintaining a certain quality. For those reasons, newly developed BoNT/A products (CORETOX®, Medytox, Inc., Republic of Korea) contained polysorbate 20, a non-human-derived excipient, to replace the HSA. However, most safety studies of polysorbate 20 have been conducted with non-invasive routes of administration, and thus there are a few studies on the safety of polysorbate 20 when administered intramuscularly. To secure the in vivo safety profile of polysorbate 20, a four-week repeated intramuscular dose toxicity study (0.02, 0.1, and 0.4 mg/kg, one injection every two weeks for a total of three injections) was conducted in 66 Sprague-Dawley (SD) rats. An intradermal irritation study was further conducted with 18 New Zealand White (NZW) rabbits. The toxicological evaluation of HSA (0.06 and 0.12 mg/kg) was also carried out as a comparative substance. Systemic and local toxicities were not observed in any of the SD rats or NZW rabbits based on clinical signs, body weight, hematology, clinical biochemistry, macroscopic findings on necropsy, histopathology of the injection site, and allergic reactions. The current study suggested that intramuscular administration of polysorbate 20 was considered to be safe at a level similar to that of HSA, which has an in vivo safety profile accumulated over the years. This provided the basis for the in vivo safety profile of polysorbate 20 administered intramuscularly and the scientific reliability of the use of polysorbate 20 as an alternative to HSA, which is used as an excipient for various pharmaceuticals in terms of its safety.
(5) Moore, J. (1984). Final report on the safety assessment of polysorbates 20, 21, 40, 60, 61, 65, 80, 81, and 85. J Am Coll Toxicol, 3, 1-82.
(6) EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), 2015. Scientific Opinion on the re‐evaluation of polyoxyethylene sorbitan monolaurate (E 432), polyoxyethylene sorbitan monooleate (E 433), polyoxyethylene sorbitan monopalmitate (E 434), polyoxyethylene sorbitan monostearate (E 435) and polyoxyethylene sorbitan tristearate (E 436) as food additives. Efsa Journal, 13(7), p.4152.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Polysorbate 20 studies" about Polysorbate-20 Review Consensus 10 by Whiz35 (11840 pt) | 2022-Sep-09 21:13 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Kim J, Kwak S, Park MS, Rhee CH, Yang GH, Lee J, Son WC, Kang WH. Safety verification for polysorbate 20, pharmaceutical excipient for intramuscular administration, in Sprague-Dawley rats and New Zealand White rabbits. PLoS One. 2021 Aug 27;16(8):e0256869. doi: 10.1371/journal.pone.0256869.
Abstract. Human serum albumin (HSA) has been widely used as a pharmaceutical excipient in Botulinum toxin serotype A (BoNT/A) products that are indicated for use in therapeutics and cosmetics. However, HSA as a human-derived material has some concerns, such as the potential risk of transmission of infectious agents, an insufficient supply, and difficulty in maintaining a certain quality. For those reasons, newly developed BoNT/A products (CORETOX®, Medytox, Inc., Republic of Korea) contained polysorbate 20, a non-human-derived excipient, to replace the HSA. However, most safety studies of polysorbate 20 have been conducted with non-invasive routes of administration, and thus there are a few studies on the safety of polysorbate 20 when administered intramuscularly. To secure the in vivo safety profile of polysorbate 20, a four-week repeated intramuscular dose toxicity study (0.02, 0.1, and 0.4 mg/kg, one injection every two weeks for a total of three injections) was conducted in 66 Sprague-Dawley (SD) rats. An intradermal irritation study was further conducted with 18 New Zealand White (NZW) rabbits. The toxicological evaluation of HSA (0.06 and 0.12 mg/kg) was also carried out as a comparative substance. Systemic and local toxicities were not observed in any of the SD rats or NZW rabbits based on clinical signs, body weight, hematology, clinical biochemistry, macroscopic findings on necropsy, histopathology of the injection site, and allergic reactions. The current study suggested that intramuscular administration of polysorbate 20 was considered to be safe at a level similar to that of HSA, which has an in vivo safety profile accumulated over the years. This provided the basis for the in vivo safety profile of polysorbate 20 administered intramuscularly and the scientific reliability of the use of polysorbate 20 as an alternative to HSA, which is used as an excipient for various pharmaceuticals in terms of its safety.
McCartan A, Mackay J, Curran D, Mrsny RJ. Modelling intramuscular drug fate in vitro with tissue-relevant biomimetic hydrogels. Int J Pharm X. 2022 Aug 13;4:100125. doi: 10.1016/j.ijpx.2022.100125.
Abstract. Parenteral administrations are a mainstay of clinical drug delivery. Intramuscular (IM) injections deposit drug directly into skeletal muscle bellies, providing rapid systemic uptake due to the highly vascularized nature of this site. The potential to inject particulate or non-aqueous materials have also made IM injections useful for long-acting formulations. These attributes have supported a plethora of medicines being approved for IM administration. Despite these many approvals across multiple pharmaceutical categories, mechanisms that control drug release from the injection site, and thus its pharmacokinetic properties, remain poorly understood....© 2022 The Authors.
Kishore RS, Kiese S, Fischer S, Pappenberger A, Grauschopf U, Mahler HC. The degradation of polysorbates 20 and 80 and its potential impact on the stability of biotherapeutics. Pharm Res. 2011 May;28(5):1194-210. doi: 10.1007/s11095-011-0385-x.
Abstract. Purpose: To study the potential impact of the degradation of Polysorbates (PS) 20 and 80 on the stability of therapeutic proteins in parenteral formulations....Conclusions: Although some degradants can potentially influence the stability of the protein (as discerned from spiking studies), degradation of polysorbates did not impact the stability of the different proteins tested in pharmaceutically relevant temperature and storage conditions.
Singh SM, Bandi S, Jones DNM, Mallela KMG. Effect of Polysorbate 20 and Polysorbate 80 on the Higher-Order Structure of a Monoclonal Antibody and Its Fab and Fc Fragments Probed Using 2D Nuclear Magnetic Resonance Spectroscopy. J Pharm Sci. 2017 Dec;106(12):3486-3498. doi: 10.1016/j.xphs.2017.08.011.
Abstract. We examined how polysorbate 20 (PS20; Tween 20) and polysorbate 80 (PS80; Tween 80) affect the higher-order structure of a monoclonal antibody (mAb) and its antigen-binding (Fab) and crystallizable (Fc) fragments, using near-UV circular dichroism and 2D nuclear magnetic resonance (NMR). ...Copyright © 2017 American Pharmacists Association®
Huille S, Brun TWVL. Industry perspective on the use and characterization of polysorbates for biopharmaceutical products Part 2: Survey report on control strategy preparing for the future. J Pharm Sci. 2022 Aug 21:S0022-3549(22)00358-6. doi: 10.1016/j.xphs.2022.08.021.
Abstract. ... The current part 2 of the survey focusses on understanding, monitoring, prediction, and mitigation of PS degradation pathways in order to propose an effective control strategy. The results of the survey and extensive cross-company discussions are put into relation with currently available scientific literature. Copyright © 2022. Published by Elsevier Inc.
Paschen CA, Klemm D, Graf T, Kopf R, Pinto C, Müller C, Bell CH, Pfaff J. Simultaneous quantification of polysorbate 20 and poloxamer 188 in biopharmaceutical formulations using evaporative light scattering detection. J Pharm Biomed Anal. 2021 Jan 5;192:113640. doi: 10.1016/j.jpba.2020.113640.
Abstract. Polysorbates and Poloxamer 188 constitute the most common surfactants used in biopharmaceutical formulations owing to their excellent protein-stabilizing properties and good safety profiles. In recent years, however, a vast number of reports concerning potential risk factors closely related with their applications, such as the accumulation of degradation products, their inherent heterogeneity and adsorption effects of proteins at silicon/oil interfaces have drawn the focus to potential alternatives....Copyright © 2020 The Author(s).
Capra P, Musitelli G, Perugini P. Wetting and adhesion evaluation of cosmetic ingredients and products: correlation of in vitro-in vivo contact angle measurements. Int J Cosmet Sci. 2017 Aug;39(4):393-401. doi: 10.1111/ics.12388.
Abstract. Objective: The aim of this work was to use the contact angle measurement in order to predict the behaviour of ingredients and finished cosmetic products on skin to improve skin feel and product texture....© 2017 Society of Cosmetic Scientists and the Société Française de Cosmétologie.
Verbrugghe M, Cocquyt E, Saveyn P, Sabatino P, Sinnaeve D, Martins JC, Van der Meeren P. Quantification of hydrophilic ethoxylates in polysorbate surfactants using diffusion H1 NMR spectroscopy. J Pharm Biomed Anal. 2010 Feb 5;51(3):583-9. doi: 10.1016/j.jpba.2009.09.025.
Abstract. ...Diffusion H1 NMR spectroscopy on a solution of polysorbate 20 in D2O revealed that only one diffusion coefficient was found for the fatty acyl part. Using the Stokes-Einstein equation, it became obvious that this diffusion behavior was caused by micelles. On the other hand, two significantly different diffusion coefficients were found for the methylene groups of ethylene oxide (EO). This indicates the presence of two distinct EO containing species in solution. ...
Moesgaard L, Reinholdt P, Nielsen CU, Kongsted J. Mechanism behind Polysorbates' Inhibitory Effect on P-Glycoprotein. Mol Pharm. 2022 Jul 4;19(7):2248-2253. doi: 10.1021/acs.molpharmaceut.2c00074.
Abstract. Much effort has been invested in the search for modulators of membrane transport proteins such as P-glycoprotein (P-gp) to improve drug bioavailability and reverse multidrug resistance in cancer. Nonionic surfactants, a class of pharmaceutical excipients, are known to inhibit such proteins, but knowledge about the exact mechanism of this inhibition is scarce. Here, we perform multiscale molecular dynamics simulations of one of these surfactants, polysorbate 20 (PS20), to reveal the behavior of such compounds on the molecular level and thereby discover the molecular mechanism of the P-gp inhibition. We show that the amphiphilic headgroup of PS20 is too hydrophobic to partition in the water phase, which drives the binding of PS20 to the amphiphilic drug-binding domain of P-gp and thereby causes the inhibition of the protein. Based on our findings, we conclude that PS20 primarily inhibits P-gp through direct binding to the drug-binding domain (DBD) from the extracellular leaflet.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2025-02-26 11:53:51 | Chemical Risk: |