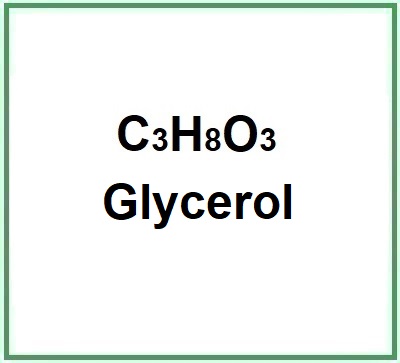
![]() Glycerol
Glycerol
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
10 pts from FRanier
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Glycerol studies" about Glycerol Review Consensus 10 by FRanier (9971 pt) | 2022-Oct-17 11:11 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Vassilev N, Malusa E, Requena AR, Martos V, López A, Maksimovic I, Vassileva M. Potential application of glycerol in the production of plant beneficial microorganisms. J Ind Microbiol Biotechnol. 2017 May;44(4-5):735-743. doi: 10.1007/s10295-016-1810-2.
Abstract. This review highlights the importance of research for development of biofertilizer and biocontrol products based on the use of glycerol for further process scale-up to industrial microbiology. Glycerol can be used successfully in all stages of production of plant beneficial microorganisms. It serves as an excellent substrate in both submerged and solid-state fermentation processes with free and immobilized microbial cells. Glycerol is also one of the most attractive formulation agents that ensures high cell density and viability including in harsh environmental conditions. Future research is discussed to make this inexpensive material a base for industrial production of plant beneficial microorganisms.
Nelson JL, Robergs RA. Exploring the potential ergogenic effects of glycerol hyperhydration. Sports Med. 2007;37(11):981-1000. doi: 10.2165/00007256-200737110-00005.
Abstract. During athletic competition or recreational pursuits, a body's hydration level can become compromised, resulting in a decrement in performance. Glycerol (1,2,3-propanetriol) has been used to induce hyperhydration in an attempt to offset the deleterious effects of dehydration. When glycerol is consumed orally, it is rapidly absorbed primarily in the small intestine. It is reported to be evenly distributed among all fluid compartments, with the exception of the cerebral spinal fluid and aqueous humour, and promotes hyperhydration by inducing an osmotic gradient. Through an increase in the kidney's medullary concentration gradient, water absorption in the nephron is enhanced. When glycerol is consumed, the plasma glycerol concentration increases in proportion to the dose ingested, which easily exceeds the glycerol saturation point resulting in urinary glycerol excretion. Thus, without supplemental glycerol ingestion, there will be a decrease in the osmotic gradient resulting in a loss of hyperhydration. The ergogenic nature of glycerol has been investigated as to its effect on fluid retention, thermoregulation, cardiovascular responses and performance. While many studies provide evidence of hyperhydration, others do not. Only two studies reviewed showed a thermoregulatory advantage. Furthermore, the preponderance of evidence neither weighed for or against cardiovascular or performance advantages. What makes one study provide favourable results while another study does not is unclear. Possible explanations may include subject characteristics, environmental factors, research design, whether fluids with or without glycerol were given during exercise, the rate at which fluids are initially given to induce hyperhydration, the time between peak hyperhydration/peak plasma glycerol concentration and the trial (i.e. exercise), the glycerol dose (i.e. 1.0 g/kg body mass) and what it is based upon, the percentage glycerol solution (i.e. 5%, 20%), the variation of time between the end of the hydration protocol and the beginning of exercise, or perhaps the intensity of exercise (fixed, variable, percentage maximum oxygen uptake). What is clear is that glycerol has the capacity to enhance fluid retention. In so doing, glycerol hyperhydration may be a logistically preferred method due to concomitant decrease in urine output and free-water clearance, which may give a performance advantage by offsetting dehydration. Future research should focus on maintaining plasma glycerol concentrations at levels necessary to maintain osmotic forces required to support continued hyperhydration. Potential benefits of glycerol should be further explored to identify the circumstances or factors that may contribute to an ergogenic effect.
Johnston DG, Alberti KG, Wright R, Blain PG. Glycerol clearance in alcoholic liver disease. Gut. 1982 Apr;23(4):257-64. doi: 10.1136/gut.23.4.257.
Abstract. Glycerol clearance was studied by a primed dose-constant infusion technique in 14 patients with alcoholic liver disease and six normal control subjects. Fasting blood glycerol concentrations were raised in the alcoholic subjects (0.09 +/- 0.01 vs 0.06 +/- 0.01 mumol/l, p less than 0.05) and glycerol clearance was impaired (24.5 +/- 1.9 vs 37.5 +/- 3.2 ml/kg/min, p less than 0.005). Endogenous production rate of glycerol and distribution space at steady state were similar in alcoholic and control subjects. The metabolic clearance rate of glycerol correlated negatively with basal glycerol concentrations. Thus tissue uptake of glycerol is impaired in liver disease. As glycerol is metabolised primarily in the liver by conversion to glucose, these data suggest a defect of gluconeogenesis in alcoholic liver disease.
Laino T, Tuma C, Curioni A, Jochnowitz E, Stolz S. A revisited picture of the mechanism of glycerol dehydration. J Phys Chem A. 2011 Apr 21;115(15):3592-5. doi: 10.1021/jp201078e.
Abstract. The dehydration mechanism of neutral glycerol in the gas phase was investigated by means of metadynamics simulations. Structures, vibrational frequencies, Gibbs free energy barriers, and rate constants at 800 K were computed for the different steps involved in the pyrolytic process. In this article, we provide a novel mechanism for the dehydration of neutral glycerol, proceeding via formation of glycidol with a barrier of 66.8 kcal/mol. The formation of glycidol is the rate limiting step of the overall decomposition process. Once formed, glycidol converts into 3-hydroxypropanal with a barrier of 49.5 kcal/mol. 3-Hydroxypropanal can decompose further into acrolein or into formaldehyde and vinyl-alcohol with barriers of 53.9 and 35.3 kcal/mol, respectively. These findings offer new insights to available experimental data based on glycerol pyrolysis studies performed with isotopic labeling and on the interpretation of the chemistry of glycerol and sugars in pyrolytic conditions.
Samra JS, Ravell CL, Giles SL, Arner P, Frayn KN. Interstitial glycerol concentration in human skeletal muscle and adipose tissue is close to the concentration in blood. Clin Sci (Lond). 1996 Jun;90(6):453-6. doi: 10.1042/cs0900453.
Abstract. 1. The suggestion that the interstitial glycerol concentration in both adipose tissue and skeletal muscle is around 3 mmol/I (Maggs DG, Jacob R, Rife F, et al. J Clin Invest 1995; 96: 370-7), rather than close to the blood concentration as previously supposed, was tested by independent methods. 2. Free glycerol was infused, as part of a triacylglycerol emulsion, into six normal subjects and the arteriovenous difference for glycerol across the forearm was measured. In addition the relative interstitial glycerol concentration in subcutaneous adipose tissue was assessed simultaneously in four of the subjects by microdialysis. 3. During glycerol infusion the arterialized glycerol concentration rose from 52 +/- 5 mumol/I to 250-300 mumol/I (P < 0.001) in a square wave fashion. The net arteriovenous difference for glycerol across the forearm changed from negative (output) to positive (uptake) (P < 0.01). In subcutaneous adipose tissue the interstitial glycerol concentration rose during glycerol infusion (P < 0.001). 4. These observations are most easily explained by the movement of glycerol from plasma to interstitial fluid down a concentration gradient. We conclude that the interstitial glycerol concentration in skeletal muscle and adipose tissue is closer to the arterial concentration than to 3 mmol/I.
Pitlick WH, Pirikitakuhlr P, Painter MJ, Wessel HB. Effect of glycerol and hyperosmolality on intracranial pressure. Clin Pharmacol Ther. 1982 Apr;31(4):466-71. doi: 10.1038/clpt.1982.61.
Abstract. Glycerol has been used in cerebral edema for hyperosmolar dehydration of brain tissue, but only empirical relationships govern this use. Since the efficacy of treatment with glycerol would likely increase with data on the relationship between drug blood levels and intracranial pressure (ICP), we examined the clinical pharmacology of the drug. Plasma samples were assayed for glycerol by a new method using gas chromatography with a flame ionization detector. Data were collected from 12 children who were in Children's Hospital of Pittsburgh (CHP) and who had cerebral edema of differing etiology that was treated with glycerol; they were monitored by intraventricular catheter. Glycerol was infused according to CHP guidelines. ICP reduction correlated with glycerol concentration and plasma concentrations of 1 to 3 mg/ml (10 to 30 mOsm/ml) were necessary to maintain an ICP below 20 torr. The relationship between osmolality and plasma glycerol level was also examined; there was good correlation between the idiogenic osmolality and drug concentration. Our studies support the clinical observations that relatively high doses of glycerol (0.2 to 1.0 gm/kg/hr), leading to plasma concentrations of 10 to 30 mOsm/l, are necessary to control ICP in patients with cerebral edema. Glycerol blood levels may be estimated from serum osmolality.
Gonçalves ARP, Ribeiro APC, Orišková S, Martins LMDRS, Cristino AF, Dos Santos RG. Glycerol Valorization-The Role of Biochar Catalysts. Molecules. 2022 Sep 1;27(17):5634. doi: 10.3390/molecules27175634.
Abstract. The conversion of renewable feedstocks into new added-value products is a current hot topic that includes the biodiesel industry. When converting vegetable oils into biodiesel, approximately 10% of glycerol byproduct is produced. Glycerol can be envisaged as a chemical platform due to its chemical versatility, as a scaffold or building block, in producing a wide range of added-value chemicals. Thus, the development of sustainable routes to obtain glycerol-based products is crucial and urgent. This certainly encompasses the use of raw carbonaceous materials from biomass as heterogeneous acid catalysts. Moreover, the integration of surface functional groups, such as sulfonic acid, in carbon-based solid materials, makes them low cost, exhibiting high catalytic activity with concomitant stability. This review summarizes the work developed by the scientific community, during the last 10 years, on the use of biochar catalysts for glycerol transformation.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Glycerol Review Consensus 10 by FRanier (9971 pt) | 2024-Jan-30 15:41 |
| Read the full Tiiip | (Send your comment) |
Glycerol is a natural three-atom trivalent, hygroscopic alcohol found in the human body, discovered in 1779 by a Swedish scientist named K. W. Scheele while experimenting with a chemical reaction between olive oil and lead monoxide later identified as glycerol.
The name defines the structure of the molecule:
- The name 'glycerol' refers to the three hydroxyl groups (-OH) that make up the molecule.
The synthesis process takes place in different steps:
- Glycerol is often a by-product of the soap production process. When fats or oils (which are triglycerides) are reacted with a strong base such as sodium hydroxide, a process called saponification, the triglycerides are broken down into their component fatty acids and glycerol.
- The fatty acids can react with the base to form soap, while the glycerol can be separated.
- The glycerol thus produced is crude and contains impurities, so it must be purified. This is typically done through a distillation process.
- The end result of this process is pure glycerol.
Glycerin is the trade name when the percentage of glycerol is 95%, but the names are mostly used to identify the same product. In other words: the pure chemical component is called glycerol, while glycerine contains about 95 per cent glycerol.
It occurs as an odourless, colourless to brown viscous liquid. Insoluble in chloroform, ether, carbon disulphide, benzene, oil. Miscible with ethanol and water. Can absorb moisture from the air as well as other volatile chemicals such as hydrogen sulphide, hydrogen cyanide and sulphur dioxide.

What it is used for and where it is used
There are approximately 1,600 applications for glycerol.
Medical
Since 1961, glycerol has been used in the treatment of intracranial hypertension, glaucoma, cerebral oedema resulting from acute ischaemic stroke, and rehydration to improve tolerance to many heat-related stressors involving exercise. Glycerol has been used as a lubricant in cases of dry mouth, in cataract surgery and acts as an osmotic dehydrating agent that has effects on brain metabolism by decreasing intracranial pressure at certain doses, reducing intraocular pressure in glaucoma and has proved very useful during neurosurgical procedures on the brain (1).
Food
- Hygroscopic agent, sweetener and solvent. Labelled E422 on the European food additives list as an emulsifier. Glycerol can be converted to monolaurate glycerol used in the food industry as a preservative, surfactant, and emulsifier. Emulsifiers have the property of reducing interfacial tension and also directly influence the stability, sensory properties and surface tension of sunscreens by modulating their filmometric performance. Glycerol is used to make glycerol mono oleate, a product of glycerolysis of camellia oil with the help of lipase as a catalyst that has been used in the production of ice cream.
Military
- Preparation of nitroglycerine, an explosive. Anticorrosive agent
Pharmaceutical
- Syrups, suppository ointments as humectant, lubricant. Inserted in medicinal tablets as a humectant and flow agent.
Cosmetics
Its water solubility, hygroscopicity and hydrophilicity characteristics give glycerol humectant and moisturising capabilities that are of great importance in most topical cosmetic applications. By improving skin texture, it acts as a skin barrier. In cleansers, shampoos and other cosmetic products, it is included in the formula with the aim of moisturising and softening the skin, preserving it from ageing or roughness. It is also used in toothpastes as a humectant and to protect gum and tooth tissue.
- Denaturant. The ionic or polar molecules of this ingredient included in formulations that interact with protein groups, modulate the properties of the solution to suit specific needs.
- Hair conditioning agent. A significant number of ingredients with specific and targeted purposes may co-exist in hair shampoo formulations: cleansers, conditioners, thickeners, matting agents, sequestering agents, fragrances, preservatives, special additives. However, the indispensable ingredients are the cleansers and conditioners as they are necessary and sufficient for hair cleansing and manageability. The others act as commercial and non-essential auxiliaries such as: appearance, fragrance, colouring, etc. Hair conditioning agents have the task of increasing shine, manageability and volume, and reducing static electricity, especially after treatments such as colouring, ironing, waving, drying and brushing. They are, in practice, dispersants that may contain cationic surfactants, thickeners, emollients, polymers. The typology of hair conditioning agents includes: intensive conditioners, instant conditioners, thickening conditioners, drying conditioners. They can perform their task generally accompanied by other different ingredients.
- Humectant. Hygroscopic compound used to minimise water loss in the skin and to prevent it from drying out by facilitating faster and greater absorption of water into the stratum corneum of the epidermis. The epidermis is the most superficial of the three layers that make up human skin (epidermis, dermis and hypodermis) and is the layer that maintains hydration in all three layers. In turn, the epidermis is composed of five layers: horny, the most superficial, granular, spinous, shiny, and basal. Humectants have the ability to retain the water they attract from the air in the stratum corneum and have the function of moisturising the skin. They are best used before emollients, which are oil-based.
- Oral care agent. This ingredient can be placed in the oral cavity to improve and/or maintain oral hygiene and health, to prevent or improve a disorder of the teeth, gums, mucous membrane.
- Perfuming. Unlike fragrance, which can also contain slightly less pleasant or characteristic odours, the term perfume indicates only very pleasant fragrances.
- Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
- Skin protectant. It creates a protective barrier on the skin to defend it from harmful substances, irritants, allergens, pathogens that can cause various inflammatory conditions. These products can also improve the natural skin barrier and in most cases more than one is needed to achieve an effective result.
- Solvent. It is the substance for dissolving or dispersing surfactants, oils, dyes, flavourings, bactericidal preservatives in solution.
- Viscosity control agent. It controls and adapts viscosity to the required level for optimal chemical and physical stability of the product and dosage in gels, suspensions, emulsions, solutions.
Textile industry
- Finishing techniques, co-solvent, dispersant, moisture absorbent. Wetting agent, hygroscopic agent, anti-shrinkage treatment of fabrics.
Coatings
- Alkyd resin (modified thermosetting polyester), polyester resin, epoxy resin and glycidyl ether.
and also: papermaking, in leather, photography, metalworking, rubber, printing and dyeing, antifreeze in lubricants and the petroleum sector.
Sport
- The use of orally or intravenously administered glycerol has been prohibited.
The most relevant studies on this ingredient have been selected with a summary of their contents:
Typical optimal commercial product characteristics Glycerol
| Appearance | Colorless to brown colored liquid |
| Boiling Point | 290.0±0.0 °C at 760 mmHg |
| Melting Point | 17.8℃(18.17℃,20℃) |
| Density | 1.3±0.1 g/cm3 1.26331 (20 ºC) |
| pH | 5.5-8 (25℃, 5M in H2O) |
| Flash Point | 160.0±0.0 °C |
| Relative vapor density (air = 1) | 3.1 |
| Viscosity (20 ºC) : 1412 mpa. S (25 ºC) | 945 mpa. S |
| Surface tension (20 ºC) | 63.3 mN/m |
| Saturated vapor pressure (kPa) | 0.4 (20 ºC) |
| Ignition temperature | 370° |
| Volume expansion coefficient/K - 1 | 0.000615 |
| Saponification equivalent | ≤5mg/kg |
| Heavy metals | ≤2mg/kg |
| Refractive Index | 1.47547~1.4730 |
 |  |
 |  |
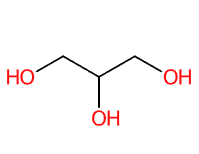
- Molecular Formula : C3H8O3 C3H5(OH)3 CH2OH-CHOH-CH2OH
- Linear Formula : HOCH2CH(OH)CH2OH
- PMolecular Weight : 92.094 g/mol
- Exact Mass 92.047340
- CAS : 56-81-5 8043-29-6 25618-55-7 8013-25-0
- UNII PDC6A3C0OX
- EC Number: 200-289-5
- MDL number: MFCD00004722
- PubChem Substance ID: 24895216
- DSSTox Substance ID DTXSID9020663 DTXSID4020662
- IUPAC propane-1,2,3-triol
- InChI=1S/C3H8O3/c4-1-3(6)2-5/h3-6H,1-2H2
- InChl Key PEDCQBHIVMGVHV-UHFFFAOYSA-N
- SMILES C(C(CO)O)O
- ChEBI 17754
- ICSC 0624
- NSC 759633 9230
- RTECS MA8050000
- NCI C29077
Synonyms:
- propan-1,2,3-triol
- Glycerin
- Glycerine
- Glycyl alcohol
- 1,2,3-trihydroxypropane
- 1,2,3-Propanetriol
- Synthetic glycerin
- Polyglycerine
References_____________________________________________________________________
(1) Frank MS, Nahata MC, Hilty MD. Glycerol: a review of its pharmacology, pharmacokinetics, adverse reactions, and clinical use. Pharmacotherapy. 1981 Sep-Oct;1(2):147-60. doi: 10.1002/j.1875-9114.1981.tb03562.x.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Natural Main substances: Last update: 2022-10-17 09:12:36 | Chemical Risk: |