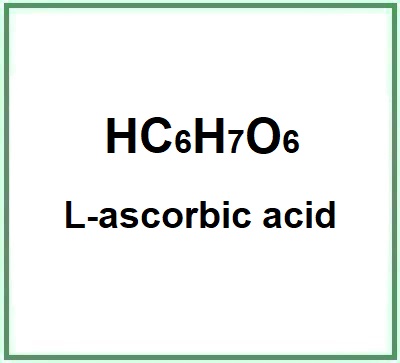
![]() L-ascorbic acid
L-ascorbic acid
Rating : 9
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Cons:
To be taken in controlled quantity (1)10 pts from Al222
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "L-Ascorbic Acid studies" about L-ascorbic acid Review Consensus 10 by Al222 (20707 pt) | 2022-Oct-19 18:22 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Pappenberger G, Hohmann HP. Industrial production of L-ascorbic Acid (vitamin C) and D-isoascorbic acid. Adv Biochem Eng Biotechnol. 2014;143:143-88. doi: 10.1007/10_2013_243.
Abstract. L-ascorbic acid (vitamin C) was first isolated in 1928 and subsequently identified as the long-sought antiscorbutic factor. Industrially produced L-ascorbic acid is widely used in the feed, food, and pharmaceutical sector as nutritional supplement and preservative, making use of its antioxidative properties. Until recently, the Reichstein-Grüssner process, designed in 1933, was the main industrial route. Here, D-sorbitol is converted to L-ascorbic acid via 2-keto-L-gulonic acid (2KGA) as key intermediate, using a bio-oxidation with Gluconobacter oxydans and several chemical steps. Today, industrial production processes use additional bio-oxidation steps with Ketogulonicigenium vulgare as biocatalyst to convert D-sorbitol to the intermediate 2KGA without chemical steps. The enzymes involved are characterized by a broad substrate range, but remarkable regiospecificity. This puzzling specificity pattern can be understood from the preferences of these enyzmes for certain of the many isomeric structures which the carbohydrate substrates adopt in aqueous solution. Recently, novel enzymes were identified that generate L-ascorbic acid directly via oxidation of L-sorbosone, an intermediate of the bio-oxidation of D-sorbitol to 2KGA. This opens the possibility for a direct route from D-sorbitol to L-ascorbic acid, obviating the need for chemical rearrangement of 2KGA. Similar concepts for industrial processes apply for the production of D-isoascorbic acid, the C5 epimer of L-ascorbic acid. D-isoascorbic acid has the same conformation at C5 as D-glucose and can be derived more directly than L-ascorbic acid from this common carbohydrate feed stock.
Elmore AR. Final report of the safety assessment of L-Ascorbic Acid, Calcium Ascorbate, Magnesium Ascorbate, Magnesium Ascorbyl Phosphate, Sodium Ascorbate, and Sodium Ascorbyl Phosphate as used in cosmetics. Int J Toxicol. 2005;24 Suppl 2:51-111. doi: 10.1080/10915810590953851.
Abstract. L-Ascorbic Acid, Calcium Ascorbate, Magnesium Ascorbate, Magnesium Ascorbyl Phosphate, Sodium Ascorbate, and Sodium Ascorbyl Phosphate function in cosmetic formulations primarily as antioxidants. Ascorbic Acid is commonly called Vitamin C. Ascorbic Acid is used as an antioxidant and pH adjuster in a large variety of cosmetic formulations, over 3/4 of which were hair dyes and colors at concentrations between 0.3% and 0.6%. For other uses, the reported concentrations were either very low (<0.01%) or in the 5% to 10% range. ...These data coupled with an absence of reports in the clinical literature of Ascorbic Acid sensitization strongly support the safety of these ingredients.
Capponi PC, Murri D, Pernice C. Topical L-Ascorbic Acid Formulation for a Better Management of Non-Melanoma Skin Cancer: Perspective for Treatment Strategies. Pharmaceutics. 2021 Aug 4;13(8):1201. doi: 10.3390/pharmaceutics13081201.
Abstract. L-ascorbic acid, is a well-known molecule, sometimes used as antioxidant for skin care. Nonetheless, few studies have taken in account its utility as topical treatment for non-melanoma skin vancer. Non-melanoma skin cancer includes basal cell carcinoma and squamous cell carcinoma and is widespread worldwide with an increasing incidence. The purpose of this paper is to analyze the characteristics of L-ascorbic acid topical formulation, its percutaneous absorption and biochemical mechanism, focusing on its anti-cancer properties. In particular, it will be described how the pH and the concentration of the formulation are able to influence its distribution in the skin and tissues. We will report, the current knowledge on the pharmacokinetic aspects of L-ascorbic acid that allows us to reconsider it in the light of its ability to act as a prodrug and as an anticancer agent. Lastly, a short review with the aim to find any evidence of a possible clinical use of L-ascorbic acid for the treatment of non-melanoma skin cancer was made.
Humbert PG, Haftek M, Creidi P, Lapière C, Nusgens B, Richard A, Schmitt D, Rougier A, Zahouani H. Topical ascorbic acid on photoaged skin. Clinical, topographical and ultrastructural evaluation: double-blind study vs. placebo. Exp Dermatol. 2003 Jun;12(3):237-44. doi: 10.1034/j.1600-0625.2003.00008.x.
Abstract. Vitamin C is known for its antioxidant potential and activity in the collagen biosynthetic pathway. Photoprotective properties of topically applied vitamin C have also been demonstrated, placing this molecule as a potential candidate for use in the prevention and treatment of skin ageing. A topically applied cream containing 5% vitamin C and its excipient were tested on healthy female volunteers presenting with photoaged skin on their low-neck and arms in view to evaluate efficacy and safety of such treatment. A double-blind, randomized trial was performed over a 6-month period, comparing the action of the vitamin C cream vs. excipient on photoaged skin. Clinical assessments included evaluation at the beginning and after 3 and 6 months of daily treatment. They were performed by the investigator and compared with the volunteer self assessment. Skin relief parameters were determined on silicone rubber replicas performed at the same time-points. Cutaneous biopsies were obtained at the end of the trial and investigated using immunohistochemistry and electron microscopy. Clinical examination by a dermatologist as well as self-assessment by the volunteers disclosed a significant improvement, in terms of the 'global score', on the vitamin C-treated side compared with the control. A highly significant increase in the density of skin microrelief and a decrease of the deep furrows were demonstrated. Ultrastructural evidence of the elastic tissue repair was also obtained and well corroborated the favorable results of the clinical and skin surface examinations. Topical application of 5% vitamin C cream was an effective and well-tolerated treatment. It led to a clinically apparent improvement of the photodamaged skin and induced modifications of skin relief and ultrastructure, suggesting a positive influence of topical vitamin C on parameters characteristic for sun-induced skin ageing.
Bremus C, Herrmann U, Bringer-Meyer S, Sahm H. The use of microorganisms in L-ascorbic acid production. J Biotechnol. 2006 Jun 25;124(1):196-205. doi: 10.1016/j.jbiotec.2006.01.010.
Abstract. L-Ascorbic acid has been industrially produced for around 70 years. Over the past two decades, several innovative bioconversion systems have been proposed in order to simplify the long time market-dominating Reichstein method, a largely chemical synthesis by which still a considerable part of L-ascorbic acid is produced. Here, we describe the current state of biotechnological alternatives using bacteria, yeasts, and microalgae. We also discuss the potential for direct production of l-ascorbic acid exploiting novel bacterial pathways. The advantages of these novel approaches competing with current chemical and biotechnological processes are outlined.
Valpuesta V, Botella MA. Biosynthesis of L-ascorbic acid in plants: new pathways for an old antioxidant. Trends Plant Sci. 2004 Dec;9(12):573-7. doi: 10.1016/j.tplants.2004.10.002.
Abstract. The biosynthetic pathway of L-ascorbic acid (vitamin C) in plants has been established for several years. However, recent reports describe alternative pathways, revealing a more complex picture of L-ascorbic acid biosynthesis than had been expected. GDP-L-gulose and myo-inositol are proposed as new intermediates in L-ascorbic acid biosynthesis, indicating that part of the animal pathway might also be operating in plants. Enzymatic studies on the GDP-mannose- 3',5'-epimerase and L-galactono-1,4-lactone dehydrogenase suggest that they are important regulatory steps for L-ascorbic acid biosynthesis.
Pinnell SR, Yang H, Omar M, Monteiro-Riviere N, DeBuys HV, Walker LC, Wang Y, Levine M. Topical L-ascorbic acid: percutaneous absorption studies. Dermatol Surg. 2001 Feb;27(2):137-42. doi: 10.1046/j.1524-4725.2001.00264.x.
Abstract. Background: Reactive oxygen species generated by ultraviolet light result in photocarcinogenic and photoaging changes in the skin. Antioxidants protect skin from these insults. Objective: This study defines formulation characteristics for delivering L-ascorbic acid into the skin to supplement the skin's natural antioxidant reservoir....Conclusions: Delivery of topical L-ascorbic acid into the skin is critically dependent on formulation characteristics.
Elhabak M, Ibrahim S, Abouelatta SM. Topical delivery of l-ascorbic acid spanlastics for stability enhancement and treatment of UVB induced damaged skin. Drug Deliv. 2021 Dec;28(1):445-453. doi: 10.1080/10717544.2021.1886377.
Abstract. l-Ascorbic acid (LAA) is considered a powerful antioxidant that protects skin from premature aging. Maintaining the stability of vitamin C remains the biggest challenge in cosmeceuticals. Our main aim is the entrapment of high dose of vitamin C in spanlastic vesicles to provide maximum stability and efficacy. LAA-loaded spanlastics were prepared by ethanol injection method and were characterized for entrapment efficiency (EE%), particles size (PS), polydispersity index (PDI), zeta potential, deformability index (DI) and in vivo skin permeation. Selected spanlastics formula composed of span 60 and tween 60 (5:1) showed highest EE% of 89.77 ± 3.61% (w/w), high deformability of 11.13 ± 1.145 as well as good physical and chemical stability for 6 months. Improved drug penetration into stratum corneum (SC) was obtained from spanlastics compared to topical LAA solution. Quantitative real time PCR revealed that MMP2 and MMP9 levels were significantly suppressed in response to LAA spanlastics treated rats by 30.4% and 65.3%, respectively, when compared to the control group after exposure to UV irradiation. Results were confirmed by western blot analysis. Histopathological study of rat skin after UV irradiation revealed that application of LAA-loaded spanlastics provided the highest skin protection compared to UVB and LAA solution treated group which was evident by the normal thick epidermal morphology and the densely arranged dermal collagen fibers. LAA-loaded spanlastics successfully improved LAA stability, skin permeation and antioxidant protection against skin photodamage.
Cendrowski A, Królak M, Kalisz S. Polyphenols, L-Ascorbic Acid, and Antioxidant Activity in Wines from Rose Fruits (Rosa rugosa). Molecules. 2021 Apr 28;26(9):2561. doi: 10.3390/molecules26092561.
Abstract. The aim of the present study was to determine the influence of the winemaking process on the antioxidant potential and content of phenolic compounds and L-ascorbic acid in wines from the fruits of Rosa rugosa. The results obtained in this study clearly indicate that the fruits of the Rosa rugosa are a desirable raw material for the production of fruit wine. The parameters of the technological process of producing wines from rose fruits had a diversified influence on the tested quality characteristics. Aged wines contained phenolics levels of 473-958 mg/100 mL GAE. The final concentrations of ascorbic acid ranged from 61 to 155 mg/100 mL for the different variants of the wine. Wines revealed high antioxidant activity in assay with DPPH. On the basis of the obtained results, it can be assumed that all the applied variants of the winemaking process are suitable for rose fruit wine. Each variant ensured at least the stability of the antioxidant capacity.
Janda K, Kasprzak M, Wolska J. Vitamin C– structure, properties, occurrence and functions. Pomeranian J Life Sci. 2015;61(4):419-25.
Abstract. In this paper the structure of vitamin C, its physical and chemical characteristics, and occurrence are presented. The biological role of ascorbic acid, the human body’s demand for this vitamin, and its deficiency symptoms are specified.
Kędzierska M, Jamroży M, Kudłacik-Kramarczyk S, Drabczyk A, Bańkosz M, Potemski P, Tyliszczak B. Physicochemical Evaluation of L-Ascorbic Acid and Aloe vera-Containing Polymer Materials Designed as Dressings for Diabetic Foot Ulcers. Materials (Basel). 2022 Sep 15;15(18):6404. doi: 10.3390/ma15186404.
Abstract. Hydrogels belong to the group of polymers that are more and more often considered as innovative dressing materials. It is important to develop materials showing the most advantageous properties from the application viewpoint wherein in the case of hydrogels, the type and the amount of the crosslinking agent strongly affect their properties. In this work, PVP-based hydrogels containing Aloe vera juice and L-ascorbic acid were obtained via UV-induced polymerization. Next, their surface morphology (via both optical, digital and scanning electron microscope), sorption capacity, tensile strength, and elongation were characterized. Their structure was analyzed via FT-IR spectroscopy wherein their impact on the simulated body liquids was verified via regular pH and temperature measurements of these liquids during hydrogels' incubation. It was demonstrated that as the amount of the crosslinker increased, the polymer structure was more wrinkled. Next, hydrogels showed relatively smooth and only slightly rough surface, which was probably due to the fact that the modifiers filled also the outer pores of the materials. Hydrogels demonstrated buffering properties in all incubation media, wherein during the incubation the release of Aloe vera juice probably took place as evidenced by the decrease in the pH of the incubation media and the disappearance of the absorption band deriving from the polysaccharides included in the composition of this additive. Next, it was proved that as the amount of the crosslinker increased, hydrogels' crosslinking density increased and thus their swelling ratio decreased. Hydrogels obtained using a crosslinking agent with higher average molecular weight showed higher swelling ability than the materials synthesized using crosslinker with lower average molecular weight. Moreover, as the amount of the crosslinking agent increased, the tensile strength of hydrogels as well as their percentage elongation also increased.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about L-ascorbic acid Review Consensus 10 by Al222 (20707 pt) | 2023-Jul-11 14:08 |
| Read the full Tiiip | (Send your comment) |
L-ascorbic acid (ascorbic acid) is the conjugate acid of L-ascorbate and the L enantiomer of ascorbic acid. is a chemical compound, a synthesized vitamin C.
The name describes the structure of the molecule:
- L- refers to the configuration of the ascorbic acid molecule. In chemistry, the terms 'L-' and 'D-' are used to indicate the configuration of a molecule around a specific chiral centre. In the case of ascorbic acid, the 'L-' form is the one that is biologically active and used in food supplements and additives.
- Ascorbic acid. The term 'ascorbic' is derived from 'a-' (meaning 'no') and 'scorbutus' (the Latin word for scurvy), because ascorbic acid is effective in the prevention and treatment of scurvy.
The synthesis of L-ascorbic acid can be achieved by different methods, but one of the most common is the Reichstein process, which involves the following steps:
- Sorbitol fermentation. Sorbitol, a sugar alcohol, is fermented by bacteria to produce L-sorbose.
- Oxidation. L-sorbose is then oxidised to 2-keto-L-gulonic acid (2-KGA) using a microorganism or enzyme.
- Ring closure: 2-KGA is then heated under acidic conditions to cause ring closure, forming L-ascorbic acid.
What it is for and where
Medical
While vitamin C is an organic compound, ascorbic acid is the purest form of vitamin C, but it is a chemical compound, a synthesized form of vitamin C. Both have the same molecular formula C6H8O6 or Hc6H7O6
Let's talk about ascorbic acid and then we'll talk about vitamin C.
Ascorbic acid is not only an antioxidant, but has some biosynthetic peculiarities in different organisms as a co-substrate of several dioxygenases, a particular function in gene expression, the important role of regenerative enzymes, modulator of neuronal metabolism. It plays a key role in endogenous antioxidant defense and is cofactor in the production of carnitine and collagen.
Ascorbic acid insufficiency can occur in conditions of systemic oxidative stress, inflammation, sepsis, trauma.
Many clinical and laboratory studies have established the effects of ascorbic acid in the treatment and prevention of cancer, with inhibition of prostate, ovarian, and pancreatic cancer cell growth, neuroblastoma cells, and inhibition of liver cancer growth and metastasis (1). However, L-ascorbic acid is a highly unstable compound and its use in pharmaceuticals and cosmetics, particularly at higher concentrations, is limited.
Cosmetics
Antioxidant agent. Ingredient that counteracts oxidative stress and prevents cell damage. Free radicals, pathological inflammatory processes, reactive nitrogen species and reactive oxygen species are responsible for the ageing process and many diseases caused by oxidation.
Buffering agent. It is an iingredient that can bring an alkaline or acid solution to a certain pH level and prevent it from changing, in practice a pH stabiliser that can effectively resist instability and pH change.
Fragrance. It plays a decisive and important role in the formulation of cosmetic products as it provides the possibility of enhancing, masking or adding fragrance to the final product, increasing its marketability. It is able to create a perceptible pleasant odour, masking a bad smell. The consumer always expects to find a pleasant or distinctive scent in a cosmetic product.
Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
For more information:
Commercial ascorbic acid occurs as a fine white or nearly white crystalline powder or colorless crystals

Typical optimal characteristics of commercial L-ascorbic acid product
| Appearance | White or almost white, crystalline powder or colourless crystals |
| Purity | ≥99% by assay |
| Melting point | About 190℃. with decomposition |
| pH(With 5% water solution) | 2.1-2.6 |
| PH(With 2% water solution) | 2.4-2.8 |
| Specific optical rotation | 20.5℃- 21.5℃ |
| Loss on drying | ≤0.4% |
| Impurity E | ≤0.2% |
| Total impurities | ≤0.20% |
| Copper | ≤5.0ppm |
| Iron | ≤2.0ppm |
| Arsenic | ≤3.0ppm |
| Lead | ≤2.0ppm |
| Mercury | ≤1.0ppm |
| Cadmium | ≤1.0ppm |
| Heavy metals | ≤10ppm |
| Sulphated ash | ≤0.1% |
 |  |
 |  |
- Molecular Formula: C6H8O6 HC6H7O6
- Molecular Weight: 176.12 g/mol
- CAS: 50-81-7
- UNII PQ6CK8PD0R
- EC Number: 200-066-2 477-330-0
- DSSTox Substance ID: DTXSID5020106
- MDL number MFCD00064328
- PubChem Substance ID 329749065
- InChI=1S/C6H8O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h2,5,7-10H,1H2/t2-,5+/m0/s1
- InChl Key CIWBSHSKHKDKBQ-JLAZNSOCSA-N
- SMILES C(C(C1C(=C(C(=O)O1)O)O)O)O
- IUPAC (2R)-2-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxy-2H-furan-5-one
- ChEBI 29073
Synonyms:
- L(+)-Ascorbic acid
- l-ascorbic acid
- vitamin C
- Ascorbate, Sodium
Vitamin C is a water-soluble vitamin that must be consumed from external sources (mainly fruits and vegetables) because humans cannot synthesize it endogenously, whereas some animals can. From a health perspective, however, while dietary vitamin C from food has a protective association from early atherosclerosis, unfortunately, taking vitamin C supplements has an adverse association (2).
Vitamin C is is a simple low molecular weight carbohydrate and is necessary for tissue growth and repair in all parts of the body. The United States Institute of Medicine recommends a daily vitamin C intake of 75 milligrams per day for adult women and 90 milligrams per day for adult men.

Vitamin C It is used by our body to:
- make an important protein for skin, tendons, ligaments and blood vessels.
- heal wounds and scars.
- repair and maintain healthy cartilage, bones and teeth.
- In the food industry it is labeled as E300 in European food additives.
In the food industry it is labeled as E300 in European food additives.
In the human body it is used for the maintenance of the antioxidant defenses of the cell, it is a free radical scavenger. It also performs the important function of contributing to vitamin E regeneration. Vitamin C also cycles between an oxidized form (L-dehydroascorbic acid) and a reduced form (L-ascorbic acid). In addition, vitamin C is important for the absorption and metabolism of iron.
Vitamin C is one of many antioxidants. Antioxidants are substances that block some of the damage caused by free radicals.
Free radicals are formed when the body is in particular uncomfortable conditions, for example, when exposed to smoke or radiation, when ingesting too much saturated fat, etc. The accumulation of free radicals over time is largely responsible for the aging process. Free radicals can play a role in cancer, heart disease and arthritis.
The body is unable to produce vitamin C on its own and does not store it, therefore, it is important to get a certain amount of vitamin C from foods that contain it, daily.
It is used, in medicine, in the treatment of colds and flu.
In nature it is found in :
- Citrus fruits and juices, such as orange, lemon, grapefruit
- Kiwi
- Acerola
- Mango
- Papaya
- Pineapple
- Strawberries, raspberries, blueberries, cranberries
- Watermelon
- etc
References________________________________________________________________________
(1) Wan J, Zhou J, Fu L, Li Y, Zeng H, Xu X, Lv C, Jin H. Ascorbic Acid Inhibits Liver Cancer Growth and Metastasis in vitro and in vivo, Independent of Stemness Gene Regulation. Front Pharmacol. 2021 Aug 24;12:726015. doi: 10.3389/fphar.2021.726015.
(2) Agarwal M, Mehta PK, Dwyer JH, Dwyer KM, Shircore AM, Nordstrom CK, Sun P, Paul-Labrador M, Yang Y, Merz CN. Differing Relations to Early Atherosclerosis between Vitamin C from Supplements vs. Food in the Los Angeles Atherosclerosis Study: A Prospective Cohort Study. Open Cardiovasc Med J. 2012;6:113-21. doi: 10.2174/1874192401206010113.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2024-05-06 10:27:35 | Chemical Risk: |