Isopropyl Alcohol is a chemical compound, a secondary alcohol.
The name describes the structure of the molecule:
- "Isopropyl" refers to the chemical structure of the alcohol, indicating it has an isopropyl side chain.
- "Alcohol" refers to the -OH (hydroxyl group) functional group present in the molecule.
Raw materials in the production of Isopropyl Alcohol.
- Water. Used as a solvent and reaction medium.
- Propene. An unsaturated hydrocarbon which is the primary raw material for isopropyl alcohol production.
Step-by-step summary of industrial chemical synthesis process:
- Direct hydration. Propene is mixed with acidified water, and the mix is heated. The acid acts as a catalyst. This process produces a mixture of isopropyl alcohol and other compounds.
- Distillation. The mixture is then sent to distillation columns to separate isopropyl alcohol from other compounds.
- Purification. Any remaining impurities are removed through further purification processes.
What it is for and where
Medical Applications
Nausea. Isopropyl alcohol has some efficacy in the treatment of nausea and vomiting (1).
Disinfectant. Isopropanol is used to disinfect skin prior to injections and as an antiseptic for the prevention of blood culture contamination (2).
Preservative. Can be used in some medical solutions as a preservative.
Antiseptic. Found in some antiseptic solutions for cleaning minor wounds.
Commercial Applications
Cleaning. Isopropanol is commonly used as a solvent for stain removal and as a glass cleaner.
Electronics. Used for cleaning circuits and electronic components due to its ability to rapidly evaporate without leaving residues.
Antifreeze. Isopropanol is used in the production of antifreeze solutions.
Hand Sanitizers. Used as a base for some hand sanitizers.
Cosmetics
Antifoaming agent. The constituent factors for foam stabilisation are the concentration of nanoparticles and hydrophobicity. Foam, even when used in separation operations such as fractionation or flotation, can cause a decrease in density and a deterioration in quality in cosmetic products. The defoaming agent (non-polar oil, silicone oils, hydrophobic solid particles or mixtures of both) is strongly influenced by viscosity and, to an almost directly proportional extent, concentration. However, defoamers can carry an irreversible source of contamination.
Solvent. It is the substance for dissolving or dispersing surfactants, oils, dyes, flavourings, bactericidal preservatives in solution.In fact, it dissolves other components present in a cosmetic formulation. Solvents are generally liquid (aqueous and non-aqueous).
Viscosity control agent. It controls and adapts, Increasing or decreasing, viscosity to the required level for optimal chemical and physical stability of the product and dosage in gels, suspensions, emulsions, solutions.
Perfuming. Unlike fragrance, which can also contain slightly less pleasant or characteristic odours, the term perfume indicates only very pleasant fragrances. Used for perfumes and aromatic raw materials.
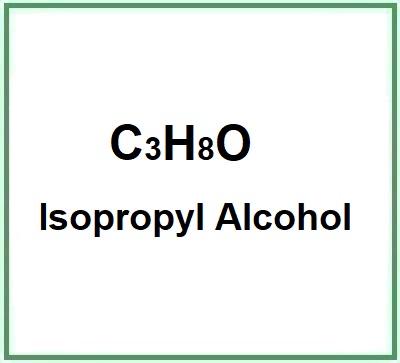
- Molecular Formula C3H8O
- Molecular Weight 60.10 g/mol
- CAS 67-63-0
- UNII ND2M416302
- EC Number 200-661-7
- DTXSID7020762
- Nikkaji J1.411G
- FEMA 2929
- RTECS NT8050000
Synonyms:
isopropanol
2-Propanol
References_____________________________________________________________________
(1) Spencer KW. Isopropyl alcohol inhalation as treatment for nausea and vomiting. Plast Surg Nurs. 2004 Oct-Dec;24(4):149-54. doi: 10.1097/00006527-200410000-00005. PMID: 15632723.
Abstract. In my practice as a recovery room nurse, I had observed anesthesiologists and nurse anesthetists wave an opened alcohol preparation pad under a patient's nose when he or she complained of nausea. When asked, "Why?'' the response often was, "Because it works.'' The following article describes the use of inhalation of isopropyl alcohol as a treatment for postoperative nausea and vomiting. Because alcohol swabs are so readily available, and certainly less expensive than some of the newer antiemetics on the market, this simple nursing intervention was worth investigating.
(2) Kiyoyama T, Tokuda Y, Shiiki S, Hachiman T, Shimasaki T, Endo K. Isopropyl alcohol compared with isopropyl alcohol plus povidone-iodine as skin preparation for prevention of blood culture contamination. J Clin Microbiol. 2009 Jan;47(1):54-8. doi: 10.1128/JCM.01425-08.
Abstract. Despite a number of studies on the efficacies of antiseptics for the prevention of blood culture contamination, it still remains unclear which antiseptic should be used. Although the combination of povidone-iodine and isopropyl alcohol has been traditionally used in many institutions, the application of povidone-iodine needs extra time, and there is little evidence that this combination could have an additive effect in reducing contamination rates. To elucidate the additive efficacy of povidone-iodine, we compared two antiseptics, 70% isopropyl alcohol only and 70% isopropyl alcohol plus povidone-iodine, in a prospective, nonrandomized, and partially blinded study in a community hospital in Japan between 1 October 2007 and 21 March 2008. All blood samples for culture were drawn by first-year residents who received formal training on collection techniques. Skin antisepsis was performed with 70% isopropyl alcohol plus povidone-iodine on all inpatient wards and with only 70% isopropyl alcohol in the emergency department. For the group of specimens from inpatient wards cultured, 13 (0.46%) of 2,797 cultures were considered contaminated. For the group of specimens from the emergency department cultured, 12 (0.42%) of 2,856 cultures were considered contaminated. There was no significant difference in the contamination rates between the two groups (relative risk, 0.90; 95% confidence interval, 0.41 to 1.98; P = 0.80). In conclusion, the use of a single application of 70% isopropyl alcohol is a sufficient and a more cost- and time-effective method of obtaining blood samples for culture than the use of a combination of isopropyl alcohol and povidone-iodine. The extremely low contamination rates in both groups suggest that the type of antiseptic used may not be as important as the use of proper technique.
![]() Isopropyl alcohol
Isopropyl alcohol