Check the ingredients!
... live healthy!


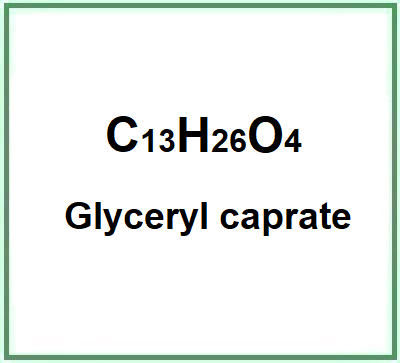
![]() Glyceryl caprate
Glyceryl caprate
Rating : 8
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Antibacterial (1)10 pts from GStream
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Glyceryl caprate studies" about Glyceryl caprate Review Consensus 10 by GStream (2740 pt) | 2022-Nov-25 10:35 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Nagata K, Takatani N, Beppu F, Abe A, Tominaga E, Fukuhara T, Ozeki M, Hosokawa M. Monocaprin Enhances Bioavailability of Fucoxanthin in Diabetic/Obese KK-Ay Mice. Mar Drugs. 2022 Jul 7;20(7):446. doi: 10.3390/md20070446.
Abstract. Fucoxanthin is a marine carotenoid found in brown seaweeds and several microalgae. It has been reported that fucoxanthin has health benefits such as anti-obesity and anti-diabetic effects. To facilitate fucoxanthin applications in the food industry, it is important to improve its low bioavailability. We attempted the combined feeding of fucoxanthin-containing seaweed oil (SO) and monocaprin in a powder diet and analyzed the fucoxanthin metabolite contents in the liver, small intestine and serum of diabetic/obese KK-Ay mice. After 4 weeks of feeding with the experimental diets, the serum fucoxanthinol concentrations of the mice fed 0.2% SO and 0.5% monocaprin were higher than those of the 0.2% SO-fed mice. Furthermore, fucoxanthinol accumulation in the liver and small intestine tended to increase in a combination diet of 0.2% SO and 0.125-0.5% monocaprin compared with a diet of 0.2% SO alone, although amarouciaxanthin A accumulation was not different among the 0.2% SO-fed groups. These results suggest that a combination of monocaprin with fucoxanthin-containing SO is an effective treatment for improving the bioavailability of fucoxanthin.
Kim YJ, Siziya IN, Hong S, Lee GY, Seo MJ, Kim YR, Yoo SH, Park CS, Seo DH. Biosynthesis of glyceride glycoside (nonionic surfactant) by amylosucrase, a powerful glycosyltransferase. Food Sci Biotechnol. 2021 Feb 6;30(2):267-276. doi: 10.1007/s10068-020-00861-0.
Abstract. Amylosucrase (ASase, E.C. 2.4.1.4) is a powerful transglycosylation enzyme that can transfer glucose from sucrose to the hydroxyl (-OH) group of various compounds. In this study, recombinant ASases from Deinococcus geothermalis (DgAS) and Bifidobacterium thermophilum (BtAS) were used to synthesize biosurfactants based on the computational analysis of predicted docking simulations. Successful predictions of the binding affinities, conformations, and three-dimensional structures of three surfactants were computed from receptor-ligand binding modes. DgAS and BtAS were effective in the synthesis of biosurfactants from glyceryl caprylate, glyceryl caprate, and polyglyceryl-2 caprate. The results of the transglycosylation reaction were consistent for both ASases, with glyceryl caprylate acceptor showing the highest concentration, as confirmed by thin layer chromatography. Furthermore, the transglycosylation reactions of DgAS were more effective than those of BtAS. Among the three substrates, glyceryl caprylate glycoside and glyceryl caprate glycoside were successfully purified by liquid chromatography-mass spectrometry (LC-MS) with the corresponding molecular weights. © The Korean Society of Food Science and Technology 2021.
Final report of the amended safety assessment of Glyceryl Laurate, Glyceryl Laurate SE, Glyceryl Laurate/Oleate, Glyceryl Adipate, Glyceryl Alginate, Glyceryl Arachidate, Glyceryl Arachidonate, Glyceryl Behenate, Glyceryl Caprate, Glyceryl Caprylate, Glyceryl Caprylate/Caprate, Glyceryl Citrate/Lactate/Linoleate/Oleate, Glyceryl Cocoate, Glyceryl Collagenate, Glyceryl Erucate, Glyceryl Hydrogenated Rosinate, Glyceryl Hydrogenated Soyate, Glyceryl Hydroxystearate, Glyceryl Isopalmitate, Glyceryl Isostearate, Glyceryl Isostearate/Myristate, Glyceryl Isostearates, Glyceryl Lanolate, Glyceryl Linoleate, Glyceryl Linolenate, Glyceryl Montanate, Glyceryl Myristate, Glyceryl Isotridecanoate/Stearate/Adipate, Glyceryl Oleate SE, Glyceryl Oleate/Elaidate, Glyceryl Palmitate, Glyceryl Palmitate/Stearate, Glyceryl Palmitoleate, Glyceryl Pentadecanoate, Glyceryl Polyacrylate, Glyceryl Rosinate, Glyceryl Sesquioleate, Glyceryl/Sorbitol Oleate/Hydroxystearate, Glyceryl Stearate/Acetate, Glyceryl Stearate/Maleate, Glyceryl Tallowate, Glyceryl Thiopropionate, and Glyceryl Undecylenate. Int J Toxicol. 2004;23 Suppl 2:55-94. doi: 10.1080/10915810490499064.
Abstract. The safety of 43 glyceryl monoesters listed as cosmetic ingredients was reviewed in a safety assessment completed in 2000. Additional safety test data pertaining to Glyceryl Rosinate and Glyceryl Hydrogenated Rosinate were received and served as the basis for this amended report. Glyceryl monoesters are used mostly as skin-conditioning agents--emollients and/or surfactant--emulsifying agents in cosmetics. The following 20 glyceryl monoesters are currently reported to be used in cosmetics: Glyceryl Laurate, Glyceryl Alginate, Glyceryl Arachidonate, Glyceryl Behenate, Glyceryl Caprylate, Glyceryl Caprylate/Caprate, Glyceryl Cocoate, Glyceryl Erucate, Glyceryl Hydroxystearate, Glyceryl Isostearate, Glyceryl Lanolate, Glyceryl Linoleate, Glyceryl Linolenate, Glyceryl Myristate, Glyceryl Oleate/Elaidate, Glyceryl Palmitate, Glyceryl Polyacrylate, Glyceryl Rosinate, Glyceryl Stearate/Acetate, and Glyceryl Undecylenate. Concentration of use data received from the cosmetics industry in 1999 indicate that Glyceryl Monoesters are used at concentrations up to 12% in cosmetic products. Glyceryl Monoesters are not pure monoesters, but are mostly mixtures with mono-, di-, and tri-esters. The purity of commercial and conventional Monoglyceride (Glyceryl Monoester) is a minimum of 90%. Glyceryl Monoesters (monoglycerides) are metabolized to free fatty acids and glycerol, both of which are available for the resynthesis of triglycerides. Glyceryl Laurate enhanced the penetration of drugs through cadaverous skin and hairless rat skin in vitro and has been described as having a wide spectrum of antimicrobial activity. A low-grade irritant response was observed following inhalation of an aerosol containing 10% Glyceryl Laurate by test animals. Glyceryl monoesters have little acute or short-term toxicity in animals, and no toxicity was noted following chronic administration of a mixture consisting mostly of glyceryl di- and mono- esters. Glyceryl Laurate did have strong hemolytic activity in an in vitro assay using sheep erythrocytes. Glyceryl Laurate, Glyceryl Isostearate, or Glyceryl Citrate/Lactate/Linoleate/Oleate were not classified as ocular irritants in rabbits. Undiluted glyceryl monoesters may produce minor skin irritation, especially in abraded skin, but in general these ingredients are not irritating at concentrations used in cosmetics. Glyceryl monoesters are not sensitizers, except that Glyceryl Rosinate and Hydrogenated Glyceryl Rosinate may contain residual rosin, which can cause allergic reactions. These ingredients are not photosensitizers. Glyceryl Citrate/Lactate/Linoleate/Oleate was not mutagenic in the Ames test system. Glyceryl Laurate exhibited antitumor activity and Glyceryl Stearate was negative in a tumor promotion assay. At concentrations higher than used in cosmetics, Glyceryl Laurate did cause moderate erythema in human repeat-insult patch test (RIPT) studies, but the other glyceryl monoesters tested failed to produce any significant positive reactions. Glyceryl Rosinate was irritating to animal skin at 50%, but did not produce sensitization in clinical tests at concentrations up to 10% and covered with semioccluded patches. There is reported use of Glyceryl Rosinate at 12%in mascara, which is somewhat higher than the concentration in the clinical testing. It was reasoned that the available data do support the safety of this use because there would be minimal contact with the skin and no occlusion. The safety of Arachidonic Acid was not documented and substantiated for cosmetic product use in an earlier safety assessment and those same safety questions apply to Glyceryl Arachidonate. Based on these data, the Cosmetic Ingredient Review (CIR) Expert Panel found that these glyceryl monoesters are safe as cosmetic ingredients in the present practices of use and concentration: except that the available data are insufficient to support the safety of Glyceryl Arachidonate. Additional data needed to support the safety of Glyceryl Arachidonate include (1) dermal absorption data; and, based on the results of the absorption studies, there may be a need for (2) immunomodulatory data; (3) carcinogenicity and photocarcinogenicity data; and (4) human irritation, sensitization, and photosensitization data.
Dolange V, Churchward CP, Christodoulides M, Snyder LAS. The Growing Threat of Gonococcal Blindness. Antibiotics (Basel). 2018 Jul 12;7(3):59. doi: 10.3390/antibiotics7030059.
Abstract. Antibiotic-resistant gonorrhea is now a reality, as well as the consequences of untreatable infections. Gonococcal eye infections result in blindness if not properly treated; they accounted for the vast majority of infections in children in homes for the blind in the pre-antibiotic era. Neisseria gonorrhoeae infects the eyes of infants born to mothers with gonorrhea and can also infect the eyes of adults. Changes in sexual practices may account for the rise in adult gonococcal eye infections, although some cases seem to have occurred with no associated genital infection. As gonorrhea becomes increasingly difficult to treat, the consequences for the treatment of gonococcal blindness must be considered as well. Monocaprin was shown to be effective in rapidly killing N. gonorrhoeae, and is non-irritating in ocular models. Repeated passage in sub-lethal monocaprin induces neither resistance in gonococci nor genomic mutations that are suggestive of resistance. Here, we show that 1 mM monocaprin kills 100% of N. gonorrhoeae in 2 min, and is equally effective against N. meningitidis, a rare cause of ophthalmia neonatorum that is potentially lethal. Monocaprin at 1 mM also completely kills Staphylococcus aureus after 60 min, and 25 mM kills 80% of Pseudomonas aeruginosa after 360 min. Previously, 1 mM monocaprin was shown to eliminate Chlamydia trachomatis in 5 min. Monocaprin is, therefore, a promising active ingredient in the treatment and prophylaxis of keratitis, especially considering the growing threat of gonococcal blindness due to antimicrobial resistance.
Subedi RK, Kang KW, Choi HK. Preparation and characterization of solid lipid nanoparticles loaded with doxorubicin. Eur J Pharm Sci. 2009 Jun 28;37(3-4):508-13. doi: 10.1016/j.ejps.2009.04.008.
Abstract. Solid lipid nanoparticles (SLN) loaded with doxorubicin were prepared by solvent emulsification-diffusion method. Glyceryl caprate (Capmul)MCM C10) was used as lipid core, and curdlan as the shell material. Dimethyl sulfoxide (DMSO) was used to dissolve both lipid and drug. Polyethylene glycol 660 hydroxystearate (Solutol)HS15) was employed as surfactant. Major formulation parameters were optimized to obtain high quality nanoparticles. The mean particle size measured by photon correlation spectroscopy (PCS) was 199nm. The entrapment efficiency (EE) and drug loading capacity (DL), determined with fluorescence spectroscopy, were 67.5+/-2.4% and 2.8+/-0.1%, respectively. The drug release behavior was studied by in vitro method. Cell viability assay showed that properties of SLN remain unchanged during the process of freeze-drying. Stability study revealed that lyophilized SLN were equally effective (p<0.05) after 1 year of storage at 4 degrees C. In conclusion, SLN with small particle size, high EE, and relatively high DL for doxorubicin can be obtained by this method.
Cho, S. W., Lee, J. S., & Choi, S. H. (2004). Enhanced oral bioavailability of poorly absorbed drugs. I. Screening of absorption carrier for the ceftriaxone complex. Journal of pharmaceutical sciences, 93(3), 612-620.
Rao, M. R., & Aghav, S. S. (2014). Spray-dried redispersible emulsion to improve oral bioavailability of itraconazole. Journal of Surfactants and Detergents, 17(4), 807-817.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Glyceryl caprate Review Consensus 10 by GStream (2740 pt) | 2023-Jan-08 17:10 |
| Read the full Tiiip | (Send your comment) |
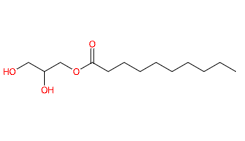
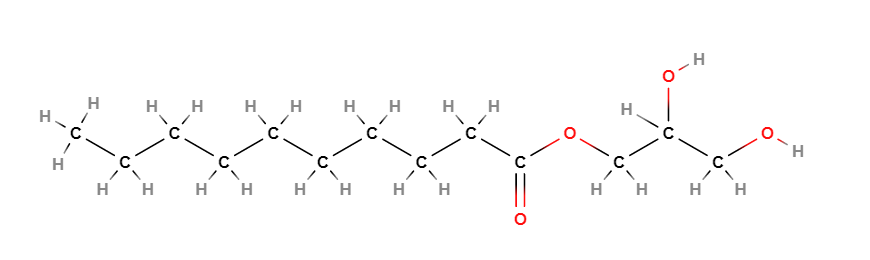
Glyceryl caprate is a chemical compound, monoglyceric monoester obtained by the esterification of glycerine and carboxylic acids (an equivalent of a carboxylic acid and an equivalent of glycerine), in this ingredient the capric acid.
Safety. The purity level of commercial monoglyceric monoester is about 90 per cent, and impurities include fatty acids, monoglyceric diesters, mono-, di- and tri-glycerides.
It appears as a white, stable powder.

What it is used for and where
Cosmetics
Surfactant with detergent function, non-ionic emulsifier, emollient, solubiliser, absorption promoter, antimicrobial, moisturiser, refractory agent. Glyceryl monoesters can be used at concentrations of up to 12% in cosmetic products and their purity is 90% so they are non-sensitising.
Surfactant - Emulsifying agent. Emulsions are thermodynamically unstable. Emulsifiers have the property to reduce the oil/water or water/oil interfacial tension, improve emulsion stability and also directly influence the stability, sensory properties and surface tension of sunscreens by modulating their filmometric performance.
Skin conditioning agent - Emollient. Emollients have the characteristic of enhancing the skin barrier through a source of exogenous lipids that adhere to the skin, improving barrier properties by filling gaps in intercorneocyte clusters to improve hydration while protecting against inflammation. In practice, they have the ability to create a barrier that prevents transepidermal water loss. Emollients are described as degreasing or refreshing additives that improve the lipid content of the upper layers of the skin by preventing degreasing and drying of the skin. The problem with emollients is that many have a strong lipophilic character and are identified as occlusive ingredients; they are oily and fatty materials that remain on the skin surface and reduce transepidermal water loss. In cosmetics, emollients and moisturisers are often considered synonymous with humectants and occlusives.
Medical
In combination with low-dose doxycycline it offers effective treatment for herpes labialis (1) and has proven to be an antimicrobial against enveloped viruses, certain Gram-positive bacteria, HSV, the yeast Candida albicans (2) and Neisseria gonorrhoeae (3). Emulsifying, dispersing, solubilising, penetrating, bacteriostatic agent in pharmaceutical products (soft gelatin capsules, coatings, films, microemulsions, suppositories).
Food
Emulsifying agent for food and viscosity modifier in flavourings, fragrances. Can remain in contact with food, dry food, paper or cardboard packaging.
Other uses
Textile industry: textiles in contact with foodstuffs.
The most relevant studies on the subject have been selected with a summary of their contents:
| Appearance | White powder |
| Flash Point | 129.6ºC |
| Density | 1.017 g/cm3 |
| Vapor Pressure | 5.96E-07mmHg at 25°C |
| Refraction Index | 1.466 |
| PSA | 66.76000 |
| LogP | 2.02350 |
| Storage | −20°C |
 |  |
 |  |
Synonyms:
Glycerol α-Monodecanoate
2,3-Dihydroxypropyl decanoate
1-Monodecanoyl Glycerol
References_____________________________________________________________________
(1) Skulason S, Holbrook WP, Thormar H, Gunnarsson GB, Kristmundsdottir T. A study of the clinical activity of a gel combining monocaprin and doxycycline: a novel treatment for herpes labialis. J Oral Pathol Med. 2012 Jan;41(1):61-7. doi: 10.1111/j.1600-0714.2011.01037.x.
(2) Thorgeirsdóttir TO, Kristmundsdóttir T, Thormar H, Axelsdóttir I, Holbrook WP. Antimicrobial activity of monocaprin: a monoglyceride with potential use as a denture disinfectant. Acta Odontol Scand. 2006 Feb;64(1):21-6. doi: 10.1080/00016350500326245.
Abstract. Monocaprin is a 1-monoglyceride of capric acid that has antimicrobial activity against enveloped viruses, certain bacteria, and the yeast Candida albicans. Solutions containing monocaprin were formulated and tested in vitro against a number of micro-organisms, including species found in the oral cavity and common pathogenic species. The antimicrobial activity of monocaprin was tested with strains growing on a surface as well as in the planktonic phase. Micro-organisms tested were: Streptococcus mutans, Candida albicans, Lactobacillus sp., Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa. Two sets of dilutions were prepared for each test strain; one to be inoculated with the micro-organism growing in the planktonic phase and the other with the same strain growing on a filter paper disk. Control solutions were also prepared to find out if any of the excipients were affecting the microbicidal effect of monocaprin. Test strains growing on the filter paper surface were less sensitive to monocaprin than the same strain growing in its planktonic phase. C. albicans was the micro-organism that was most sensitive to monocaprin, but S. mutans also showed appreciable sensitivity. The indication that monocaprin may have potential as a topical agent against Candida was tested in an open study of denture disinfection in 32 patients attending a geriatric daycare centre. A significant, but short-term, reduction in counts of Candida on the fitting surface of full dentures was observed.
(3) Dolange V, Churchward CP, Christodoulides M, Snyder LAS. The Growing Threat of Gonococcal Blindness. Antibiotics (Basel). 2018 Jul 12;7(3):59. doi: 10.3390/antibiotics7030059.
Abstract. Antibiotic-resistant gonorrhea is now a reality, as well as the consequences of untreatable infections. Gonococcal eye infections result in blindness if not properly treated; they accounted for the vast majority of infections in children in homes for the blind in the pre-antibiotic era. Neisseria gonorrhoeae infects the eyes of infants born to mothers with gonorrhea and can also infect the eyes of adults. Changes in sexual practices may account for the rise in adult gonococcal eye infections, although some cases seem to have occurred with no associated genital infection. As gonorrhea becomes increasingly difficult to treat, the consequences for the treatment of gonococcal blindness must be considered as well. Monocaprin was shown to be effective in rapidly killing N. gonorrhoeae, and is non-irritating in ocular models. Repeated passage in sub-lethal monocaprin induces neither resistance in gonococci nor genomic mutations that are suggestive of resistance. Here, we show that 1 mM monocaprin kills 100% of N. gonorrhoeae in 2 min, and is equally effective against N. meningitidis, a rare cause of ophthalmia neonatorum that is potentially lethal. Monocaprin at 1 mM also completely kills Staphylococcus aureus after 60 min, and 25 mM kills 80% of Pseudomonas aeruginosa after 360 min. Previously, 1 mM monocaprin was shown to eliminate Chlamydia trachomatis in 5 min. Monocaprin is, therefore, a promising active ingredient in the treatment and prophylaxis of keratitis, especially considering the growing threat of gonococcal blindness due to antimicrobial resistance.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances:
Last update: 2022-11-25 10:44:59 | Chemical Risk: |