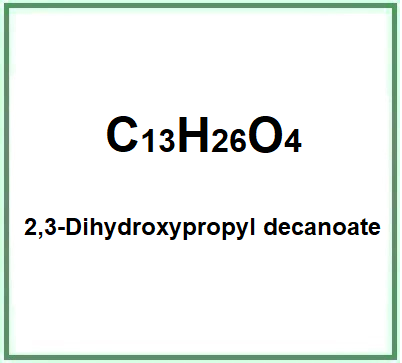
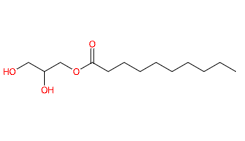
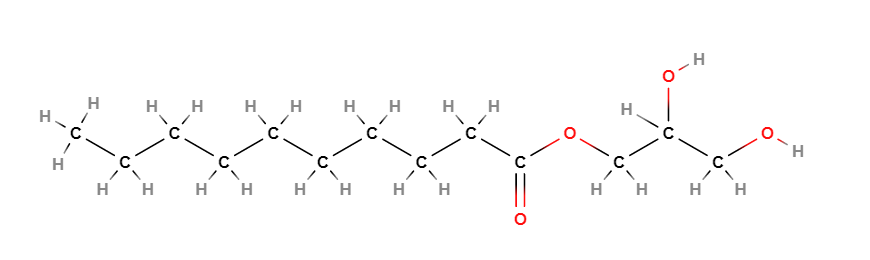
2,3-Dihydroxypropyl decanoate (Glyceryl caprate) is a glycoside glyceride, monoester lipid of glycerine and capric acid, 1-monoglyceride of capric acid.
It appears as a white, stable powder.

What it is used for and where
Cosmetics
Surfactant with detergent function, non-ionic emulsifier. solubiliser, absorption promoter, antimicrobial, moisturiser, refractory agent. Glyceryl monoesters can be used at concentrations of up to 12% in cosmetic products and their purity is 90% so they are non-sensitising.
Medical
In combination with low-dose doxycycline it offers effective treatment for herpes labialis (1) and has proven to be an antimicrobial against enveloped viruses, certain Gram-positive bacteria, HSV, the yeast Candida albicans (2) and Neisseria gonorrhoeae (3). Emulsifying, dispersing, solubilising, penetrating, bacteriostatic agent in pharmaceutical products (soft gelatin capsules, coatings, films, microemulsions, suppositories).
Food
Emulsifying agent for food and viscosity modifier in flavourings, fragrances. Can remain in contact with food, dry food, paper or cardboard packaging.
Other uses
Textile industry: textiles in contact with foodstuffs.
The most relevant studies on the subject have been selected with a summary of their contents:
Glyceryl caprate studi
| Appearance | White powder |
| Flash Point | 129.6ºC |
| Density | 1.017 g/cm3 |
| Vapor Pressure | 5.96E-07mmHg at 25°C |
| Refraction Index | 1.466 |
| PSA | 66.76000 |
| LogP | 2.02350 |
| Storage | −20°C |
- Molecular Formula CH3(CH2)8COOCH2COHHCH2OH C13H26O4
- Molecular Weight 246.34
- Exact Mass 246.18300
- CAS 26402-22-2 2277-23-8
- UNII 197M6VFC1W
- EC Number 247-667-6
- DSSTox Substance ID DTXSID2041832 DTXSID40891378 DTXSID401015098
- IUPAC 2,3-dihydroxypropyl decanoate
- InChl=1S/C13H26O4/c1-2-3-4-5-6-7-8-9-13(16)17-11-12(15)10-14/h12,14-15H,2-11H2,1H3
- InChl Key LKUNXBRZDFMZOK-UHFFFAOYSA-N
- SMILES CCCCCCCCCC(=O)OCC(CO)O
- MDL number MFCD00056656
- PubChem Substance ID 329817931
- ChEBI 75551
- SCHEMBL25295
- NCI C66185
- NACRES NA.25
- RXCUI 1366664
Synonyms:
References_____________________________________________________________________
(1) Skulason S, Holbrook WP, Thormar H, Gunnarsson GB, Kristmundsdottir T. A study of the clinical activity of a gel combining monocaprin and doxycycline: a novel treatment for herpes labialis. J Oral Pathol Med. 2012 Jan;41(1):61-7. doi: 10.1111/j.1600-0714.2011.01037.x.
(2) Thorgeirsdóttir TO, Kristmundsdóttir T, Thormar H, Axelsdóttir I, Holbrook WP. Antimicrobial activity of monocaprin: a monoglyceride with potential use as a denture disinfectant. Acta Odontol Scand. 2006 Feb;64(1):21-6. doi: 10.1080/00016350500326245.
Abstract. Monocaprin is a 1-monoglyceride of capric acid that has antimicrobial activity against enveloped viruses, certain bacteria, and the yeast Candida albicans. Solutions containing monocaprin were formulated and tested in vitro against a number of micro-organisms, including species found in the oral cavity and common pathogenic species. The antimicrobial activity of monocaprin was tested with strains growing on a surface as well as in the planktonic phase. Micro-organisms tested were: Streptococcus mutans, Candida albicans, Lactobacillus sp., Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa. Two sets of dilutions were prepared for each test strain; one to be inoculated with the micro-organism growing in the planktonic phase and the other with the same strain growing on a filter paper disk. Control solutions were also prepared to find out if any of the excipients were affecting the microbicidal effect of monocaprin. Test strains growing on the filter paper surface were less sensitive to monocaprin than the same strain growing in its planktonic phase. C. albicans was the micro-organism that was most sensitive to monocaprin, but S. mutans also showed appreciable sensitivity. The indication that monocaprin may have potential as a topical agent against Candida was tested in an open study of denture disinfection in 32 patients attending a geriatric daycare centre. A significant, but short-term, reduction in counts of Candida on the fitting surface of full dentures was observed.
(3) Dolange V, Churchward CP, Christodoulides M, Snyder LAS. The Growing Threat of Gonococcal Blindness. Antibiotics (Basel). 2018 Jul 12;7(3):59. doi: 10.3390/antibiotics7030059.
Abstract. Antibiotic-resistant gonorrhea is now a reality, as well as the consequences of untreatable infections. Gonococcal eye infections result in blindness if not properly treated; they accounted for the vast majority of infections in children in homes for the blind in the pre-antibiotic era. Neisseria gonorrhoeae infects the eyes of infants born to mothers with gonorrhea and can also infect the eyes of adults. Changes in sexual practices may account for the rise in adult gonococcal eye infections, although some cases seem to have occurred with no associated genital infection. As gonorrhea becomes increasingly difficult to treat, the consequences for the treatment of gonococcal blindness must be considered as well. Monocaprin was shown to be effective in rapidly killing N. gonorrhoeae, and is non-irritating in ocular models. Repeated passage in sub-lethal monocaprin induces neither resistance in gonococci nor genomic mutations that are suggestive of resistance. Here, we show that 1 mM monocaprin kills 100% of N. gonorrhoeae in 2 min, and is equally effective against N. meningitidis, a rare cause of ophthalmia neonatorum that is potentially lethal. Monocaprin at 1 mM also completely kills Staphylococcus aureus after 60 min, and 25 mM kills 80% of Pseudomonas aeruginosa after 360 min. Previously, 1 mM monocaprin was shown to eliminate Chlamydia trachomatis in 5 min. Monocaprin is, therefore, a promising active ingredient in the treatment and prophylaxis of keratitis, especially considering the growing threat of gonococcal blindness due to antimicrobial resistance.
![]() 2,3-Dihydroxypropyl decanoate
2,3-Dihydroxypropyl decanoate