Camphora or Camphor is a natural product, a monoterpene bicyclic ketone that is traditionally obtained by distilling the wood and bark from the Cinnamomum camphora tree that grows in Taiwan, Borneo, Asia and Japan and is also a component of the essential oil of many species of aromatic plants. It is synthesised using alpha pinene, which is obtained from the oil of turpentine through a chemical process, and natural camphor is still obtained by distilling the wood of the Cinnamomum camphora tree.
It appears in synthetic form as a white or colourless crystalline powder with a mothball-like odour. Stable. Incompatible with strong oxidising agents, organic substances, metal salts, combustible materials. Inflammable. Soluble in alcohol, chloroform, ether and other organic solvents.

What it is used for and where
Intermediate in the synthesis of aromatic chemicals.
Food
Fragrance used as an anthelmintic and preservative.
Cosmetics
Camphor oil is used as a fragrance and refreshing agent in aromatherapy products, cleansers, massage oils. Some cosmetic products containing this oil are labelled with astringent and skin-improving properties. Clinical studies have shown camphor to be a powerful anti-wrinkle agent as it induces the proliferation of primary dermal fibroblasts (1)
As with other chemical compounds included in cosmetic products, camphor is not immune to potential toxicity and this study provides the current overview of knowledge on the subject (2).
Medical
Camphor has a long history as an ancient analgesic remedy and circulatory stimulant; its fumes were used during the Black Death of 1346 and during cholera and smallpox epidemics.
Camphor produces a mild local anaesthetic, analgesic and anti-itch effect, is used in pharmaceutical products to relieve the burning and itching of insect bites and has been shown to be repellent against some insects (3). Both camphor and its essential oil have demonstrated antiviral, antimicrobial and antifungal properties (4).
This study by Cardiovascular Pharmacology, ZekaPha GmbH, Mainz-Wiesbaden, Germany, found that camphor extracted from hawthorn berries counteracts an orthostatic fall in blood pressure (5).
When taken orally, it has a mild expectorant effect, but the efficacy of the antitussive effects were found in clinical studies to be rather mixed.
Camphor has been shown to be a moderate potentiator of skin penetration (6).
Other Uses
In PVC, celluloid production, synthetic rubber, nitrocellulose, it is used as a plasticiser
In ballistite production, it is a stabilising agent.
Safety
Camphor has a high degree of toxicity (7).
For more information:
Camphor studies
Typical commercial product characteristics Synthetic camphor
| Appearance | White crystalline powder |
Boiling Point
| 207.4±0.0°C at 760 mmHg |
Melting Point
| 175-177 °C |
Flash Point
| 64.4±0.0°C |
| Density | 1.0±0.1 g/cm3 |
| PSA | 17.07000 |
| LogP | 22.13 |
| Refraction Index | 1.485 |
Vapor Pressure
| 0.2±0.4 mmHg at 25°C |
Vapor density
| 5.2 |
Water Solubility
| 0.12 g/100 mL (25 ºC) |
| Specific Optical Rotation | -2°~+5° |
| Specific Rotation (20°C) | +41° -- +44° |
| Chloride | ≤0.035% |
Reside solvent
| 0.217% |
| Safety |  |
Price
100 g $31.20
500 g $84.00
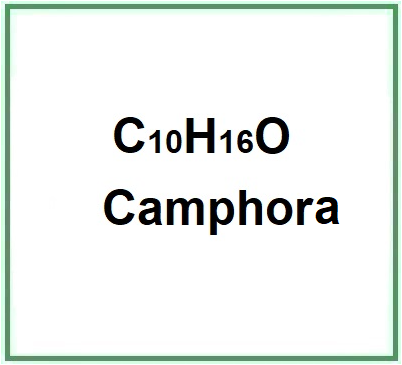
- Molecular Formula C10H16O
- Molecular Weight 152.23
- Exact Mass 152.120117
- CAS 76-22-2
- UNII 5TJD82A1ET
- EC Number 200-945-0
- DSSTox Substance ID
- IUPAC 1,7,7-trimethylbicyclo[2.2.1]heptan-2-one
- InChI=1S/C10H16O/c1-9(2)7-4-5-10(9,3)8(11)6-7/h7H,4-6H2,1-3H3
- InChl Key DSSYKIVIOFKYAU-UHFFFAOYSA-N
- SMILES CC1(C2CCC1(C(=O)C2)C)C
- MDL number MFCD00074738
- PubChem Substance ID 24848879
- ChEBI 36773
- Beilstein 1907611
- ICSC 1021
- FEMA 4513
- RTECS EX1225000 EX1260000
- UN 2717
- JECFA 1395 2199
- NACRES NA.22
- RIDADR UN 2717 4.1/PG 3
Synonyms
- 1,7,7-trimethylbicyclo[2.2.1]heptan-2-one
- 2-Oxo-bornan
- Camketone
- 2-Bornanone,2-Camphanone,D-Camphor
- Radian B
- (+)-bornan-2-one
- Bicyclo(2.2.1)heptan-2-one, 1,7,7-trimethyl-
- camphanone
- 3-Fluor-2,2-dimethyl-bicyclo<2.2.1>heptan
- (1S,4S)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one
References________________________________________________________________
(1) Tran TA, Ho MT, Song YW, Cho M, Cho SK. Camphor Induces Proliferative and Anti-senescence Activities in Human Primary Dermal Fibroblasts and Inhibits UV-Induced Wrinkle Formation in Mouse Skin. Phytother Res. 2015 Dec;29(12):1917-25. doi: 10.1002/ptr.5484.
(2) Barbaud A, Lafforgue C. Risks associated with cosmetic ingredients. Ann Dermatol Venereol. 2021 Jun;148(2):77-93. doi: 10.1016/j.annder.2020.04.027.
(3) Ojimelukwe, P. C., & Adler, C. (2000). Toxicity and repellent effects of eugenol, thymol, linalool, menthol and other pure compounds on Dinoderus bifloveatus (Coleoptera: Bostrichidae). Journal of Sustainable Agriculture and the Environment, 2(1), 47-54.
(4) Viljoen A, van Vuuren S, Ernst E, Klepser M, Demirci B, Başer H, van Wyk BE. Osmitopsis asteriscoides (Asteraceae)-the antimicrobial activity and essential oil composition of a Cape-Dutch remedy. J Ethnopharmacol. 2003 Oct;88(2-3):137-43. doi: 10.1016/s0378-8741(03)00191-0.
(5) Belz GG, Loew D. Dose-response related efficacy in orthostatic hypotension of a fixed combination of D-camphor and an extract from fresh crataegus berries and the contribution of the single components. Phytomedicine. 2003;10 Suppl 4:61-7. doi: 10.1078/1433-187x-00303.
(6) Ramesh, G., Vamshi Vishnu, Y., Kishan, V., & Rao, Y. M. (2007). Studies on the influence of penetration enhancers on in vitro permeation of carvedilol across rat abdominal skin. Current Trends in Biotechnology and Pharmacy, 1(1), 62-69.
(7) Siegel E, Wason S. Camphor toxicity. Pediatr Clin North Am. 1986 Apr;33(2):375-9. doi: 10.1016/s0031-3955(16)35008-8.
![]() Camphora
Camphora