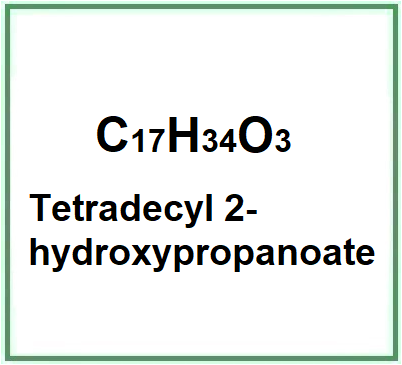
Tetradecyl 2-hydroxypropanoate (Myristyl lactate) is the ester produced by a catalyzed reaction of either natural or synthetic myristyl alcohol and lactic acid. The ester produced by the reaction is washed to remove any catalyst and unreacted lactic acid. The final product is further washed with alkali and neutralized.
The name describes the structure of the molecule
- Tetradecyl - Refers to tetradecyl alcohol, a long-chain fatty alcohol.
- 2-Hydroxypropanoate - Another name for lactic acid.
Description of raw materials used in production
- Tetradecyl alcohol - A long-chain fatty alcohol that can be obtained from plant or animal sources.
- Lactic acid (2-hydroxypropanoic acid) - Can be produced synthetically or derived from bacterial fermentation of carbohydrates.
Synthesis process
- Esterification reaction - Tetradecyl alcohol reacts with lactic acid in the presence of an acid catalyst to form Tetradecyl 2-Hydroxypropanoate.
It appears as a clear to yellowish liquid or in solid form as a white powder. In liquid form at room temperature and in solid form below room temperature.

What it is used for and where
Cosmetics
It acts as an emollient, lubricating, texturising agent with properties of imparting shine and silkiness to hair and skin. Has a degree of water repellency.
Skin conditioning agent - Emollient. Emollients have the characteristic of enhancing the skin barrier through a source of exogenous lipids that adhere to the skin, improving barrier properties by filling gaps in intercorneocyte clusters to improve hydration while protecting against inflammation. In practice, they have the ability to create a barrier that prevents transepidermal water loss. Emollients are described as degreasing or refreshing additives that improve the lipid content of the upper layers of the skin by preventing degreasing and drying of the skin. The problem with emollients is that many have a strong lipophilic character and are identified as occlusive ingredients; they are oily and fatty materials that remain on the skin surface and reduce transepidermal water loss. In cosmetics, emollients and moisturisers are often considered synonymous with humectants and occlusives.
Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
Dosage: at 0.5-2% levels and maximum concentration 25%.
Applications
- Emollient - Tetradecyl 2-hydroxypropanoate acts as an emollient, helping to keep the skin soft and smooth.
- Skin Conditioning - Can enhance the appearance and feel of the skin, making it look more moisturized and supple.
- Solvent - It can act as a solvent in some cosmetic products, helping to solubilize other ingredients.
- Texture Enhancers - Can be used to adjust the texture and viscosity of creams, lotions, and other skincare products.
- Partially Natural Origin - Since it's derived from tetradecyl alcohol and lactic acid, it can be considered of partially natural origin.
Safety
It has not demonstrated toxicity, irritation or sensitisation at doses formulated as safe in cosmetic products.
Myristyl lactate studies
Typical commercial product characteristics Myristyl lactate
| Appearance | White powder |
| Boiling Point | 330ºC at 760 mmHg |
| Flash Point | 147.1ºC |
| Density | 0.922 g/cm3 |
| Vapor Pressure | 1.26E-05mmHg at 25°C |
| Refraction Index | 1.453 |
| PSA | 46.53000 |
| LogP | 4.61150 |
| Purity | 99.8% |
| Assay | 99.8% |
| Dryness | 0.19% |
Heavy Metal
| <10ppm |
Sulfated Ash
| 0.009% |
Residue On Ignition
| 0.03% |
| Specific Gravity | 0.892-0.904 |
| Titer | 11°C-14°C |
| Acid value | 3 max |
| Ester value | 166-185 |
| Saponification value | 166-185 |
| Iodine value | 1.0 max |
- Molecular Formula C17H34O3
- Molecular Weight 286.4
- Exact Mass 286.25100
- CAS 1323-03-1
- UNII 1D822OC34X
- EC Number 215-350-1
- DSSTox Substance ID DTXSID50862630
- IUPAC tetradecyl 2-hydroxypropanoate
- InChl=1S/C17H34O3/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-20-17(19)16(2)18/h16,18H,3-15H2,1-2H3
- InChl Key BORJONZPSTVSFP-UHFFFAOYSA-N
- SMILES CCCCCCCCCCCCCCOC(=O)C(C)O
- MDL number
- PubChem Substance ID
- RXCUI 1363720
- HS Code 2918110000
Synonyms:
- Tetradecyl lactate
- Myristyl lactate
![]() Tetradecyl 2-hydroxypropanoate
Tetradecyl 2-hydroxypropanoate