![]() Tartaric acid
Tartaric acid
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Hair care (1)10 pts from Ark90
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Tartaric acid studies" about Tartaric acid Review Consensus 10 by Ark90 (12432 pt) | 2022-Nov-16 19:51 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Fujimura M, Sakamoto S, Kamio Y, Saito M, Miyake Y, Yasui M, Matsuda T. Cough threshold to inhaled tartaric acid and bronchial responsiveness to methacholine in patients with asthma and sino-bronchial syndrome. Intern Med. 1992 Jan;31(1):17-21. doi: 10.2169/internalmedicine.31.17.
Abstract. To evaluate the effect of chronic airway inflammation on cough sensitivity and bronchial responsiveness, we measured the cough threshold to tartaric acid and bronchial responsiveness to methacholine (PC20-FEV1) in 13 asthmatic, 13 bronchitic (sino-bronchial syndrome) and 49 healthy non-atopic subjects. All subjects were non-smokers. The geometric mean value of the cough threshold was 9.55, 5.62 and 12.3% in asthmatic, bronchitic and normal subjects, respectively. The value in bronchitic subjects was significantly (p less than 0.02) lower than that in normal subjects. The geometric mean value of PC20-FEV1 in asthmatic subjects (0.63 mg/ml) was significantly lower than those in bronchitic (8.7 mg/ml) (p less than 0.01) and normal subjects (21.4 mg/ml) (p less than 0.01). There was no correlation between cough threshold and PC20-FEV1 values [correlation coefficient (r) = 0.06, p greater than 0.1]. These results indicate that cough sensitivity is potentiated by chronic airway inflammation in bronchitis but not in asthma and suggest that cough sensitivity and bronchial responsiveness may be independently potentiated by different mechanisms resulting from chronic airway inflammation.
Danilewicz JC. Role of tartaric and malic acids in wine oxidation. J Agric Food Chem. 2014 Jun 4;62(22):5149-55. doi: 10.1021/jf5007402.
Abstract. Tartaric acid determines the reduction potential of the Fe(III)/Fe(II) redox couple. Therefore, it is proposed that it determines the ability of Fe to catalyze wine oxidation. The importance of tartaric acid was demonstrated by comparing the aerial oxidation of 4-methylcatechol (4-MeC) in model wine made up with tartaric and acetic acids at pH 3.6. Acetic acid, as a weaker Fe(III) ligand, should raise the reduction potential of the Fe couple. 4-MeC was oxidized in both systems, but the mechanisms were found to differ. Fe(II) readily reduced oxygen in tartrate model wine, but Fe(III) alone failed to oxidize the catechol, requiring sulfite assistance. In acetate model wine the reverse was found to operate. These observations should have broad application to model systems designed to study the oxidative process in foods and other beverages. Consideration should be given to the reduction potential of metal couples by the inclusion of appropriate ligands.
Domínguez-López I, Parilli-Moser I, Arancibia-Riveros C, Tresserra-Rimbau A, Martínez-González MA, Ortega-Azorín C, Salas-Salvadó J, Castañer O, Lapetra J, Arós F, Fiol M, Serra-Majem L, Pintó X, Gómez-Gracia E, Ros E, Lamuela-Raventós RM, Estruch R. Urinary Tartaric Acid, a Biomarker of Wine Intake, Correlates with Lower Total and LDL Cholesterol. Nutrients. 2021 Aug 22;13(8):2883. doi: 10.3390/nu13082883.
Abstract. Postmenopausal women are at higher risk of developing cardiovascular diseases due to changes in lipid profile and body fat, among others. The aim of this study was to evaluate the association of urinary tartaric acid, a biomarker of wine consumption, with anthropometric (weight, waist circumference, body mass index (BMI), and waist-to-height ratio), blood pressure, and biochemical variables (blood glucose and lipid profile) that may be affected during the menopausal transition. This sub-study of the PREDIMED (Prevención con Dieta Mediterránea) trial included a sample of 230 women aged 60-80 years with high cardiovascular risk at baseline. Urine samples were diluted and filtered, and tartaric acid was analyzed by liquid chromatography coupled to electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS). Correlations between tartaric acid and the study variables were adjusted for age, education level, smoking status, physical activity, BMI, cholesterol-lowering, antihypertensive, and insulin treatment, total energy intake, and consumption of fruits, vegetables, and raisins. A strong association was observed between wine consumption and urinary tartaric acid (0.01 μg/mg (95% confidence interval (CI): 0.01, 0.01), p-value < 0.001). Total and low-density lipoprotein (LDL) cholesterol were inversely correlated with urinary tartaric acid (-3.13 μg/mg (-5.54, -0.71), p-value = 0.016 and -3.03 μg/mg (-5.62, -0.42), p-value = 0.027, respectively), whereas other biochemical and anthropometric variables were unrelated. The results suggest that wine consumption may have a positive effect on cardiovascular health in postmenopausal women, underpinning its nutraceutical properties.
Spiller GA, Story JA, Furumoto EJ, Chezem JC, Spiller M. Effect of tartaric acid and dietary fibre from sun-dried raisins on colonic function and on bile acid and volatile fatty acid excretion in healthy adults. Br J Nutr. 2003 Oct;90(4):803-7. doi: 10.1079/bjn2003966.
Abstract. Sun-dried raisins are a source of dietary fibre and tartaric acid. The effects of tartaric acid on colon function have not been the focus of extensive research. The purpose of the present study was to evaluate the effects of dietary fibre and tartaric acid from sun-dried raisins on colon function and on faecal bile acid and short-chain fatty acid (SCFA) excretion in healthy adults. Thirteen healthy subjects were fed 120 g sun-dried raisins/d or 5 g cream of tartar (equivalent to the tartaric acid in 120 g sun-dried raisins)/d for 9 weeks, divided into 3-week cycles. The experimental diets were fed in a crossover design after an initial control period. Faeces were collected for the last 4 d of each cycle for analysis of SCFA and bile acids. Intestinal transit time decreased from 42 h on the baseline diet to 31 h on cream of tartar (P<0.1) and to 28 h on sun-dried raisins (P<0.05). Faeces were softer on both sun-dried raisins and cream of tartar, but sun-dried raisins increased faecal wet weight (P<0.05), while cream of tartar did not. Sun-dried raisins caused significant reductions from baseline values in total bile acid concentration (from 1.42 (SD 1.03) to 1.09 (SD 0.76) mg/g, P<0.05), whereas cream of tartar did not (1.40 (SD 1.06) mg/g). Sun-dried raisins also significantly reduced the lithocholic (LC):deoxylithocholic acid (DC) ratio (from 1.63 (SD 0.85) to 1.09 (SD 0.50), P<0.02), whereas cream of tartar reduced the ratio, but to a lesser extent (1.29 (SD 0.79), NS). Both faecal bile acids and the LC:DC ratio are indicators of reduced risk for colon cancer. Sun-dried raisins increased total SCFA excretion (from 5.6 (SD 3.4) to 7.6 (SD 3.0) g/4 d, P<0.05), which remained unchanged with cream of tartar (5.6 (SD 3.0) g/4 d). Both sun-dried raisins and cream of tartar appear to be good stool softeners and to shorten intestinal transit time, although the fibre in sun-dried raisins has the added benefit of increasing faecal weight. Both sun-dried raisins and cream of tartar modulate the composition of faecal bile acids and SCFA in a way that has potential health benefits.
Bánhegyi DF, Fogassy E, Madarász J, Pálovics E. Optical Resolution of Two Pharmaceutical Bases with Various Uses of Tartaric Acid Derivatives and Their Sodium Salts: Racemic Ephedrine and Chloramphenicol Base. Molecules. 2022 May 13;27(10):3134. doi: 10.3390/molecules27103134.
Abstract. The optically active dibenzoyltartaric acid, tartaric acid, and its sodium salts were successfully applied to the optical resolution of (1R,2S)(1S,2R)-2-(methylamino)-1-phenylpropan-1-ol (EPH) and (1R,2R)(1S,2S)-2-amino-1-(4-nitrophenyl)propane-1,3-diol (AD) as resolving agents. It was observed that both compounds' resolution using a mixture of salts of quasi-racemic resolving agents showed a change in chiral recognition under the same conditions compared to the result of the use of the single enantiomeric resolving agent. The changes are followed by detailed analytical (XRD, FTIR, and DSC) studies. Meanwhile, the DASH indexing software package was also tested on powder XRD patterns of pure initial materials and intermediate salt samples of high diastereomeric excess.
Xuan J, Feng Y. Enantiomeric Tartaric Acid Production Using cis-Epoxysuccinate Hydrolase: History and Perspectives. Molecules. 2019 Mar 5;24(5):903. doi: 10.3390/molecules24050903.
Abstract. Tartaric acid is an important chiral chemical building block with broad industrial and scientific applications. The enantioselective synthesis of l(+)- and d(-)-tartaric acids has been successfully achieved using bacteria presenting cis-epoxysuccinate hydrolase (CESH) activity, while the catalytic mechanisms of CESHs were not elucidated clearly until very recently. As biocatalysts, CESHs are unique epoxide hydrolases because their substrate is a small, mirror-symmetric, highly hydrophilic molecule, and their products show very high enantiomeric purity with nearly 100% enantiomeric excess. In this paper, we review over forty years of the history, process and mechanism studies of CESHs as well as our perspective on the future research and applications of CESH in enantiomeric tartaric acid production.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Tartaric acid Review Consensus 10 by Ark90 (12432 pt) | 2023-Jul-08 12:24 |
| Read the full Tiiip | (Send your comment) |
Tartaric acid is a white crystalline organic acid and an important chiral chemical found in nature in many fruits such as grapes, bananas and tamarinds.
The name 'tartaric acid' is derived from the Latin word 'tartarum', which refers to 'tartar', the sediment that forms inside wine barrels, also known as tartaric cream when wine is refined. This sediment is one of the main sources of tartaric acid.
The synthesis of tartaric acid in the laboratory can be achieved by different methods, but a common method involves the following steps:
- Fermentation: Tartaric acid can be obtained by fermenting grape juice. During the fermentation process, the tartaric acid present in the grape juice remains in the wine.
- Crystallisation: After the fermentation process, the wine is left to rest in the cold. Tartaric acid is less soluble in cold solutions, so it crystallises from the wine when it cools. These crystals are often called 'wine diamonds'.
- Purification: The crystals are collected and then purified to obtain tartaric acid. This can be done by recrystallisation, in which the crystals are dissolved in hot water and then allowed to crystallise again when the solution cools.
- Drying and packaging: The purified tartaric acid is then dried and packaged for use.
In nature, tartaric acid is synthesised by many plants, particularly grapes, through a series of biochemical reactions as part of their metabolic processes.
It appears as a white crystalline powder.

What it is used for and where
Medical
Tartaric acid has a wide range of applications in pharmaceuticals. It is a well-known cough test for assessing cough function (1), improves colon function and excretion of faecal bile acids and short-chain fatty acids.
Negative contrast agent in double-contrast X-rays, as a reagent for resolving basic racemic compounds.
Cosmetics
Buffering agent. It is an iingredient that can bring an alkaline or acid solution to a certain pH level and prevent it from changing, in practice a pH stabiliser that can effectively resist instability and pH change.
Fragrance. It plays a decisive and important role in the formulation of cosmetic products as it provides the possibility of enhancing, masking or adding fragrance to the final product, increasing its marketability. The consumer always expects to find a pleasant or distinctive scent in a cosmetic product.
A recent discovery by a Japanese pharmaceutical company, however not yet confirmed by clinical studies, could help baldness sufferers. Tartaric acid, according to this manufacturer, could increase substances that promote hair growth. In this study by researchers at the University of Konkuk, Japan, tartaric acid was able to maintain the colour of dyed hair and protect hair more efficiently than succinic acid as well as increase the tensile strength of hair, decrease hair porosity and smoothen the hair surface (2).
Food
It is inexpensive, readily available and widely used in beverages and other foods. In wine, tartaric acid, the main organic acid molecule responsible for the acidity of wine, together with tannin improves its colour (3). An acidic agent in food additives labelled with the number E334 in the list of European food additives with the function of an acidity regulator.
Other uses
It is one of the main acids found in wine and plays a key role in winemaking. Tartaric acid has a sour taste and is also used as an antioxidant and flavoring in food.
Catalyst in resin finishing in polyester fabrics, sulphur removal, electroplating, acid pickling, complexing agent, screening or chelating agent in chemical analysis, reducing agent in the chemical production of mirrors, imaging agent in photography, pH value adjuster in the production of oryzanol, polishing agent in metals, metal cleaner.
Safety
Tartaric acid (E 334), sodium tartrates (E 335), potassium tartrates (E 336), sodium potassium tartrate (E 337) and calcium tartrate (E 354) are authorised as food additives according to Regulation (EC) No 1333/2008 on food additives.
The Panel established a group ADI for l(+)‐tartaric acid and tartrates (E 334‐337 and E 354) of 240 mg/kg bw per day, expressed as tartaric acid, by applying the total uncertainty factor of 10 to the reference point of 3,100 mg sodium tartrate/kg bw per day (the highest dose tested), that is approximately 2,440 mg tartaric acid/kg bw per day. Accordingly, the current ADI of 30 mg/kg bw per day is withdrawn (4).
The most relevant studies and their abstracts have been selected for further study:
Typical commercial product characteristics Tartaric acid
| Appearance | White crystal powder |
| Boiling Point | 399.3±42.0°C at 760 mmHg |
| Melting Point | 170-172°C |
| Flash Point | 210ºC |
| Density | 1.76 |
| Refraction Index | 1.586 |
| Vapor Pressure | 0.0±2.1 mmHg at 25°C |
| Vapor Density | 5.18 |
| PSA | 115.06000 |
| LogP | -1.43 |
| Specific rotation | +12°~12.8° |
| Loss on drying | 0.2 max |
| Heavy metals Pb | 0.001 max |
| Residue on ignition | 0.05 max |
| Oxalate C2O4 | 0.035 max |
| Sulphate SO4 | |
| As | 0.0003 max |
| Cl | 0.01 max |
| Chemical Safety |  |
 |  |
 |  |
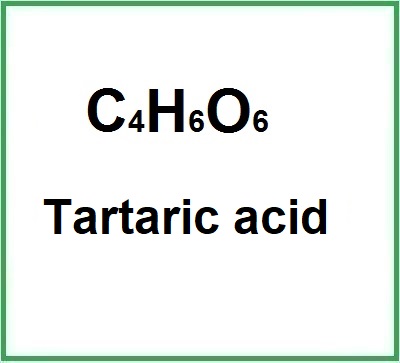
- Molecular Formula C4H6O6 H2C4H4O6
- Linear Formula: HO2CCH(OH)CH(OH)CO2H
- Molecular Weight: 150.086 g/mol
- Exact Mass 150.016434
- CAS: 87-69-4
- UNII 4J4Z8788N8 W4888I119H
- EC Number: 205-695-6 201-766-0
- DSSTox Substance ID DTXSID8023632 DTXSID5046986
- IUPAC (2R,3R)-2,3-dihydroxybutanedioic acid
- InChl=1S/C4H6O6/c5-1(3(7)8)2(6)4(9)10/h1-2,5-6H,(H,7,8)(H,9,10)/t1-,2-/m1/s1
- InChl Key FEWJPZIEWOKRBE-JCYAYHJZSA-N
- SMILES C(C(C(=O)O)O)(C(=O)O)O
- RTECS WW7875000
- FEMA Number: 3044
- JECFA 621
- PubChem Substance ID 329751224
- MDL number: MFCD00064207
- Beilstein Registry Number: 1725147
- NCI C47744
- RXCUI 37578
Synonyms:
- DL-Tartaric acid
- L-tartaric acid
- Racemic acid
- Racemic tartaric acid
- 2,3-Dihydroxysuccinic acid
- 2,3-Dihydroxybutanedioic acid
- DL-Tartrate
- Paratartaric aicd
- Paratartaric acid
- Threaric acid
References____________________________________________
(1) Ohno T, Tanaka N, Fujimori M, Okamoto K, Hagiwara S, Hojo K, Shigematsu T, Sugi T, Kanazawa H, Kunieda K, Fujishima I. Cough-Inducing Method Using a Tartaric Acid Nebulizer for Patients with Silent Aspiration. Dysphagia. 2022 Jun;37(3):629-635. doi: 10.1007/s00455-021-10313-4.
(2) Jung, Y. J., & Lee, S. H. (2021). Effect of Post-Treatment using Succinic Acid and Tartaric Acid During Dyeing Process on Hair Conditions. Journal of Convergence for Information Technology, 11(12), 221-228.
(3) Effect of sodium sulfite, tartaric acid, tannin, and glucose on rheological properties, release of aroma compounds, and color characteristics of red wine. Wang H, Ni ZJ, Ma WP, Song CB, Zhang JG, Thakur K, Wei ZJ. Food Sci Biotechnol. 2018 Oct 15;28(2):395-403. doi: 10.1007/s10068-018-0492-0.
(4) EFSA Panel on Food Additives and Flavourings (FAF); Younes M, Aquilina G, Castle L, Engel KH, Fowler P, Frutos Fernandez MJ, Fürst P, Gürtler R, Gundert-Remy U, Husøy T, Mennes W, Shah R, Waalkens-Berendsen I, Wölfle D, Boon P, Tobback P, Wright M, Aguilera J, Rincon AM, Tard A, Moldeus P. Re-evaluation of l(+)-tartaric acid (E 334), sodium tartrates (E 335), potassium tartrates (E 336), potassium sodium tartrate (E 337) and calcium tartrate (E 354) as food additives. EFSA J. 2020 Mar 11;18(3):e06030. doi: 10.2903/j.efsa.2020.6030.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Natural Main substances: Last update: 2019-05-06 16:45:23 | Chemical Risk: |