Nonanoic acid (Pelargonic acid) is a natural, medium-chain, nine-carbon-atom saturated fatty acid found in edible plants, in the exometabolome of the tomato, in some rhododendrons (Rhododendron mucronulatum), in buttercups (Staphisagria macrosperma), in plants of the Malvaceae family, and in animals.

The name describes the structure of the molecule:
- Nonanoic indicates a carbon chain with nine carbon atoms. The suffix "-ic" indicates that the acid has the -COOH functional group at the end of the carbon chain.
- acid refers to a substance that has the ability to donate protons (H+ ions) in a solution and has a pH less than 7. Organic acids, like nonanoic acid, contain the -COOH functional group.
Description of raw materials used in production:
- Fatty acids source. Nonanoic acid can be derived from natural sources, such as vegetable oils, animal fats, or can be synthesized from petrochemical sources. These sources provide the fatty acids needed for the production of nonanoic acid.
Summary of the industrial synthesis process step-by-step:
- Source selection. The raw material source for nonanoic acid, such as vegetable oils or petrochemicals, is selected based on availability and cost-effectiveness.
- Extraction (for natural sources). If nonanoic acid is derived from natural sources, such as vegetable oils, an extraction process is employed to isolate the fatty acids.
- Hydrolysis (for natural sources). In the case of natural sources, the extracted fats or oils undergo hydrolysis, which breaks down the triglycerides into glycerol and free fatty acids, including nonanoic acid.
- Chemical synthesis (for petrochemical sources). If nonanoic acid is synthesized from petrochemical sources, it involves a series of chemical reactions, such as oxidation or decarboxylation, to obtain the desired nonanoic acid.
- Purification. The crude nonanoic acid obtained from either extraction or chemical synthesis is purified to remove impurities, such as residual solvents, by-products, or other contaminants.
- Distillation. The purified nonanoic acid undergoes distillation to separate it from any remaining impurities and obtain a higher purity product.
- Characterization. The synthesized nonanoic acid is characterized through various analytical techniques to confirm its identity, purity, and physical properties.
- Packaging and distribution. The final product, nonanoic acid, is packaged in suitable containers and distributed for use in various industries, according to their specific requirements.
It appears as a colourless oily liquid insoluble in water, soluble in ether, ethanol and chloroform.

What it is used for and where
Cosmetics
Pelargonic acid and its esters act as fairly safe and non-toxic skin-conditioning agents, but skin penetration is possible. They have proven to be non-irritating and non-sensitising to human skin in laboratory tests, but some formulations may improve penetration into the stratum corneum.
Surfactant - Cleansing agent
Cosmetic products used to cleanse the skin utilise the surface-active action that produces a lowering of the surface tension of the stratum corneum, facilitating the removal of dirt and impurities.
Surfactant - Emulsifying agent
Emulsions are thermodynamically unstable. Emulsifiers have the property to reduce the oil/water or water/oil interfacial tension, improve emulsion stability and also directly influence the stability, sensory properties and surface tension of sunscreens by modulating their filmometric performance.
Medical
A commonly recognised safe antifungal agent and disinfectant (GRAS), it also possesses antimicrobial activity (1) and is used as an aqueous antimicrobial treatment to preserve fresh produce including the possible internalisation of pathogens by plant tissue (2). It is registered with the EPA (3).
Food
Fragrance. Used in the preparation of coconut and berry flavours. FDA-approved food additive. Credited with antimicrobial properties
Other uses
Together with other chemicals, such as glyphosate (Roundup) it is an ingredient in herbicide formulations, herbicides.
Synthetic lubricant, plasticiser, paint dryer.
Safety
It has not demonstrated toxicity to humans at low quantities.
The most relevant studies on this acid have been selected with a summary of the contents:
Pelargonic acid studies
Caratteristiche tipiche del prodotto commerciale Pelargonic acid
| Appearance | Colorless oily liquid |
| Boiling Point | 269°C 516.20°F litre |
| Melting Point | 9°C 48.20°F litre |
| Flash Point | 100°C 212°F |
| Density | 0.906 g/mL at 25°C |
| Refraction Index | n20/D 1.432(lit.) |
| Vapor Pressure | <0.1 mm Hg at 20°C |
| Vapor density | 5.5 |
| PSA | 37.30000 |
| LogP | 2.82160 |
| Purity | ≥99% |
| Water | ≤0.5% |
| Total acid | 97.5%-100% |
Acid value
| 345.00-355.00 mg KOH/g |
| Iodine value | 0.00-0.50 gl2/100g |
| Iron | 0-2 ppm |
| Transmittance | 95-100% 440nm |
| Transmittance | 99-100% 5500nm |
| Storage | 2-8°C |
| Shelf Life | 2 years |
| Chemical Safety |  |
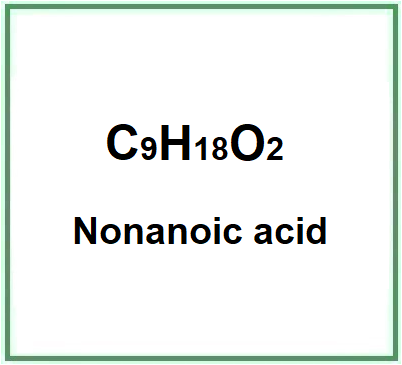
- Molecular Formula C9H18O2
- Linear Formula CH3(CH2)7COOH
- Molecular Weight 158.24
- Exact Mass 158.13100
- CAS 112-05-0
- UNII 97SEH7577T
- EC Number 203-931-2
- DSSTox Substance ID DTXSID3021641 DTXSID5028326 DTXSID9028887
- IUPAC nonanoic acid
- InChl=1S/C9H18O2/c1-2-3-4-5-6-7-8-9(10)11/h2-8H2,1H3,(H,10,11)
- InChl Key FBUKVWPVBMHYJY-UHFFFAOYSA-N
- SMILES CCCCCCCCC(=O)O
- MDL number MFCD00004433
- PubChem Substance ID 24897735
- ChEBI 29019
- JECFA 102
- FEMA 2784
- RTECS RA6650000
- NCI C82932
- Beilstein 1752351
- NACRES NA.25
Synonyms
- Pelargonic acid
- Nonanoic acid
- Nonoic acid
- 1-octanecarboxylic acid
- n-Nonanoic acid
References_____________________________________________________________________
(1) Dev Kumar G, Mis Solval K, Mishra A, Macarisin D. Antimicrobial Efficacy of Pelargonic Acid Micelles against Salmonella varies by Surfactant, Serotype and Stress Response. Sci Rep. 2020 Jun 24;10(1):10287. doi: 10.1038/s41598-020-67223-y.
(2) Herdt J., Feng H. Aqueous antimicrobial treatments to improve fresh and fresh-cut produce safety. In: Fan X., Niemira B.A., Doona C.J., Feeherry F.E., Gravani R.B., editors. Microbial Safety of Fresh Produce. Blackwell Publishing and the Institute of Food Technologists; Ames, IA, USA: 2009. pp. 169–190.
(3) Biopesticides Fact Sheet for Pelargonic acid (epa.gov)
![]() Nonanoic acid
Nonanoic acid