Glycine is the only α-amino acid of proteins without a stereocenter. Amino acids play a key metabolic function in the human body and are constituents of proteins.
This name is derived from the Greek word 'glykys', meaning sweet. It was so named because of its sweet taste.
It is generally not synthesised in the laboratory, but is produced naturally by the body and is found in various foods. However, for commercial production, glycine can be synthesised by different methods. A common method is Strecker amino acid synthesis, which involves the following steps:
- Amine formation. An aldehyde or ketone is reacted with ammonia or a primary or secondary amine to form an imine.
- Reaction with cyanide. An imine is reacted with hydrogen cyanide (HCN) to form an α-aminonitrile.
- Hydrolysis. The α-aminonitrile is then hydrolysed, under acidic or basic conditions, to obtain an amino acid, in this case glycine.
What it is for and where
Amino acids play a key metabolic function in the human body and are building blocks of proteins.
α-amino acids that have similar physical structures undergo similar changes with regard to solubility in water/ethanol mixtures, and technologies to separate α-amino acids from industrial residues, which may not even be innocuous, are constantly being improved. However, many data on the solubility in water-ethanol and ethanol of some α-amino acids are contradictory or even lacking, and the effects of ethanol on the solubility of amino acids may be different. Overall, the scientific literature considers that α-amino acids do not pose significant problems for human health when taken orally, except in people with certain genetic diseases.
Medical
Substantial experimental evidence in the literature supports that free glycine may play a role in protecting tissues against insults such as ischaemia, hypoxia and reperfusion (1).
This study illustrates the role of small molecules, including glycine, in hypoxia (low tissue oxygen levels). It also discusses the importance of oxygen partial pressure in human tissue (2).
Food
Amino acids as food additives they perform different functions: preservatives, flavour enhancers, food supplements and more.
Food safety: amino acid α generally considered safe.
Cosmetics
Amino acids together with their salts are used in cosmetics as conditioning agents for both hair and skin (e.g. as moisturisers and other similar functions).
Antistatic agent. Static electricity build-up has a direct influence on products and causes electrostatic adsorption. The antistatic ingredient reduces static build-up and surface resistivity on the surface of the skin and hair.
Buffering agent. It is an iingredient that can bring an alkaline or acid solution to a certain pH level and prevent it from changing, in practice a pH stabiliser that can effectively resist instability and pH change.
Hair conditioning agent. A significant number of ingredients with specific and targeted purposes may co-exist in hair shampoo formulations: cleansers, conditioners, thickeners, matting agents, sequestering agents, fragrances, preservatives, special additives. However, the indispensable ingredients are the cleansers and conditioners as they are necessary and sufficient for hair cleansing and manageability. The others act as commercial and non-essential auxiliaries such as: appearance, fragrance, colouring, etc. Hair conditioning agents have the task of increasing shine, manageability and volume, and reducing static electricity, especially after treatments such as colouring, ironing, waving, drying and brushing. They are, in practice, dispersants that may contain cationic surfactants, thickeners, emollients, polymers. The typology of hair conditioning agents includes: intensive conditioners, instant conditioners, thickening conditioners, drying conditioners. They can perform their task generally accompanied by other different ingredients.
Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
Cosmetic safety: amino acid α generally considered safe when formulated to be non-irritant.

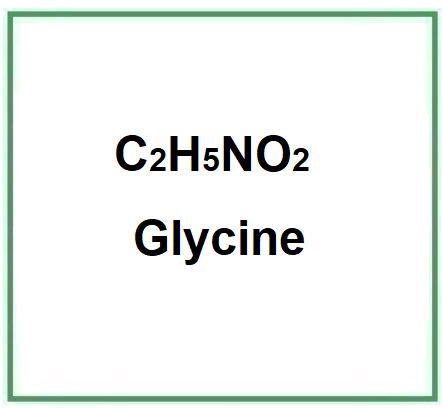
- Molecular Formula C2H5NO2
- Molecular Weight 75.07 g/mol
- CAS 56-40-6
- UNII TE7660XO1C
- EC Number 200-272-2
References_____________________________________________________________________
(1) Hall JC. Glycine. JPEN J Parenter Enteral Nutr. 1998 Nov-Dec;22(6):393-8. doi: 10.1177/0148607198022006393.
Abstract. Glycine consists of a single carbon molecule attached to an amino and a carboxyl group. Its small size helps it to function as a flexible link in proteins and allows for the formation of helices, an extracellular signaling molecule, recognition sites on cell membranes and enzymes, a modifier of molecular activity via conjugation and glycine extension of hormone precursors, and an osmoprotectant. There is substantial experimental evidence that free glycine may have a role in protecting tissues against insults such as ischemia, hypoxia, and reperfusion. This impressive catalogue of functions makes an interesting contrast with glycine's perceived metabolic role as a nonessential amino acid. Glycine interconverts with serine to provide a mechanism for the transfer of activated one-carbon groups. Glycine has just been viewed as a convenient source of nitrogen to add to solutions of nutrients. Although this may have unexpected benefits when such solutions are used in clinical practice, it does raise the specter of a possible confounding effect in experiments when glycine is added to control solutions to make them isonitrogenous.
(2) Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med. 2011 Jun;15(6):1239-53. doi: 10.1111/j.1582-4934.2011.01258.x.
![]() Glycine
Glycine