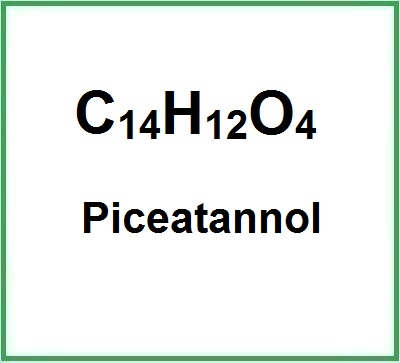
![]() Piceatannol
Piceatannol
Rating : 10
Pros:
Possible anti-cancer (1) Anti-inflammatory (1) Antimicrobial (1)Piceatannol (trans-3, 4,3 ', 5'-tetrahydroxystilbene) is a polyphenol that has been discovered in grapes, red wine and rhubarb (Rheum undulatum).It has important properties:
anticancer
inflammatory
antimicrobial
Recent studies have found its ability to suppress a broad spectrum of tumor cells (1) (2).Molecular Formula: C14H12O4Molecular Wei... (Read the full Tiiip)
8 pts from A_Partyns
| Evaluate | Where is this found? |
| "Descrizione" about Piceatannol Review Consensus 8 by A_Partyns (12948 pt) | 2019-Oct-30 21:30 |
Piceatannol (trans-3, 4,3 ', 5'-tetrahydroxystilbene) is a polyphenol that has been discovered in grapes, red wine and rhubarb (Rheum undulatum).It has important properties:
anticancer
inflammator ...
| Read the full Tiiip | (Send your comment) |
Read other Tiiips about this object in __Italiano (1)
Component type: Natural Main substances: Last update: 2013-02-01 18:07:12 | Chemical Risk: |