![]() Lysozyme
Lysozyme
Rating : 8
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Oral hygiene. Anti-plaque (1) Cognitive decline (1) Anti-inflammatory (1) Antibacterial (1)9 pts from FRanier
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about Lysozyme Review Consensus 9 by FRanier (9971 pt) | 2023-Jul-17 21:36 |
| Read the full Tiiip | (Send your comment) |
Lysozyme is a lytic enzyme-type protein found in the human body (liver, spleen. saliva) eggs and other animal tissues, a peptide with antimicrobial activity.
The name describes the structure of the molecule:
- "Lyso-" indicates that the enzyme has the ability to lyse, or break down, bacterial cell walls.
- "-zyme" indicates that it is an enzyme, which is a class of proteins with catalytic activity.
Therefore, the name "lysozyme" indicates an enzyme that has the ability to lyse bacterial cell walls.
Lysozyme can be produced both through a natural synthesis process and synthetically through industrial chemical synthesis. Here is a summary of the two processes, first in Italian and then in English:
Natural synthesis:
Lysozyme is naturally produced by various organisms, including humans. It is synthesized in organs such as salivary glands, lacrimal glands, and white blood cells. Synthesis occurs at the cellular level through DNA and RNA transcription and translation mechanisms, leading to the formation of the lysozyme enzyme.
Industrial chemical synthesis:
The industrial chemical synthesis process of lysozyme involves the following steps:
- Isolation of lysozyme. Lysozyme can be isolated from natural sources, such as chicken eggs, which are one of the main commercial sources of lysozyme.
- Extraction. Lysozyme is extracted from chicken eggs or other natural sources using chemical extraction or purification techniques.
- Purification. The extracted sample undergoes purification processes such as filtration, chromatography, or precipitation to obtain pure lysozyme.
- Formulation. Pure lysozyme is formulated into solutions or powders used for medical applications.
Industrially it appears in the form of a white powder.

What it is used for and where
Food
Ingredient included in the list of European food additives as E1105 with a preservative function and is added to cheeses (e.g. grana padano) to maintain their integrity and to counteract Clostridia spp. bacteria.
Medical
Lysozyme has several applications in the medical field. It is used as a natural antimicrobial agent, as it can break down the cell walls of gram-positive bacteria. It is also used as a preservative in some pharmaceutical and food products. Additionally, lysozyme is being studied for its immunomodulatory and anti-inflammatory properties and may have potential use in the therapy of inflammatory diseases and as a biomarker for certain pathological conditions.
It acts as an antioxidant, anti-inflammatory and antibacterial agent especially against Gram-positive and Gram-negative bacteria (1), acts as a protective agent in Alzheimer's disease (2), in the treatment of infections by Staffilococcus Aureus bacteria (3) and is an effective substitute for chlorhexidine and benzidine in the treatment of acute pharyngitis (4).
Cosmetics
Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
 |  |
 |  |
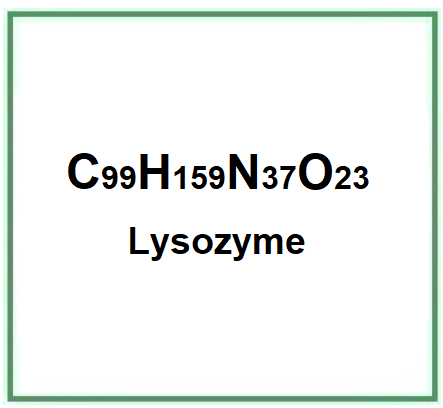
- Molecular Formula C99H159N37O23
- Molecular Weight 2235.6
- CAS 9001-63-2 235-747-3
- UNII
- EC number 232-620-4
References_________________________________________________________________________
(1) Vilcacundo R, Méndez P, Reyes W, Romero H, Pinto A, Carrillo W. Antibacterial Activity of Hen Egg White Lysozyme Denatured by Thermal and Chemical Treatments. Sci Pharm. 2018 Oct 30;86(4):E48. doi: 10.3390/scipharm86040048.
Abstract. The aim of this study was to increase the antibacterial spectrum of modified hen egg white lysozyme (HEWL) with thermal and chemical treatments against Gram-negative bacteria. The antibacterial activity of heat-denatured HEWL and chemical denatured HEWL against Gram-negative and Gram-positive bacteria was evaluated in 15 h of incubation tests. HEWL was denatured by heating at pH 6.0 and pH 7.0 and chemical denaturing was carried out for 1.0, 1.5, 2.0, and 4.0 h with DL-Dithiothreitol (DTT). HEWL modified by thermal and chemical treatments was characterized using the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) electrophoresis method. Heat-denatured HEWL lytic activity against Micrococcus lysodeikticus lessened with increasing temperature and time of incubation with the chemical agent (DTT). The loss of lytic activity in modified HEWL suggests that the mechanism of action of the antibacterial activity is not dependent on the lytic activity. Thermal and chemical treatments of HEWL enabled the production of oligoforms and increased antibacterial activity over a wider spectrum. Heat-denatured HEWL at pH 6.0 and chemically-denatured HEWL increased the HEWL antibacterial spectrum against Gram-negative bacteria (Escherichia coli ATCC 25922). HEWL at 120 °C and pH 6.0 (1.0 mg/mL) inhibited 78.20% of the growth of E. coli. HEWL/DTT treatment for 4.0 h (1.0 mg/mL) inhibited 68.75% of the growth E. coli. Heat-denatured HEWL at pH 6.0 and pH 7.0 and chemically-denatured HEWL (1.0, 1.5, 2.0, and 4.0 h with DTT) were active against Gram-positive bacteria (Staphylococcus carnosus CECT 4491T). Heat-denatured and chemical-denatured HEWL caused the death of the bacteria with the destruction of the cell wall. LIVE/DEAD assays of fluorescent dye stain of the membrane cell showed membrane perturbation of bacteria after incubation with modified HEWL. The cell wall destruction was viewed using electron microscopy. The results obtained in this study suggest that heat-denatured HEWL at pH 6.0 and chemical-denatured HEWL treatments increase the HEWL antibacterial activity against Gram-negative bacteria.
(2) Helmfors L, Boman A, Civitelli L, Nath S, Sandin L, Janefjord C, McCann H, Zetterberg H, Blennow K, Halliday G, Brorsson AC, Kågedal K. Protective properties of lysozyme on β-amyloid pathology: implications for Alzheimer disease. Neurobiol Dis. 2015 Nov;83:122-33. doi: 10.1016/j.nbd.2015.08.024.
(3) Chen, L.L., Shi, W.P., Zhang, T.D., Zhou, Y.Q., Zhao, F.Z., Ge, W.Y., Jin, X.Q., Lin, W.J., Guo, W.H. and Yin, D.C., 2022. Antibacterial activity of lysozyme-loaded cream against MRSA and promotion of scalded wound healing. International Journal of Pharmaceutics, 627, p.122200.
Abstract. Staphylococcus aureus (S. aureus) infection, especially its drug-resistant bacterial infection, is a great challenge often faced by clinicians and patients, and it is also one of the most important threats to public health. Finding a safe and effective antibacterial agent is of great significance for the prevention and treatment of S. aureus infection. Lysozyme is known to have antibacterial effects against Gram-positive bacteria including S. aureus. Here, high-quality lysozyme with a purity of more than 99% and an activity of more than 60, 000 U/mg was prepared from egg white, which showed excellent antibacterial activity against three strains of S. aureus, especially against MRSA. Furthermore, an antibacterial cream loaded with lysozyme was prepared and tested in scald wound healing. The lysozyme-loaded cream exhibited the effect of preventing wound infection and promoting wound healing on scalds, and no toxicity was found in animal organs. Overall, lysozyme showed great application potential in the prevention and treatment of infections caused by S. aureus and scalded wound healing. The most remarkable discovery in this work is the unexpectedly powerful inhibitory effect of lysozyme on the drug-resistant bacterial, especially MRSA, which is usually very difficult to deal with using normal antibacterial drugs.
(4) Golac-Guzina, N., Novaković, Z., Sarajlić, Z., Šukalo, A., Džananović, J., Glamočlija, U., Kapo, B., Čordalija, V. and Mehić, M., 2019. Comparative study of the efficacy of the lysozyme, benzydamine and chlorhexidine oral spray in the treatment of acute tonsillopharyngitis-results of a pilot study. Acta Medica Academica, 48(2), pp.140-146.
Abstract. Objective. Lysozyme is a natural antimicrobial and immunomodullatory enzyme, which is produced as a host response to infectious agents. The objective of this study was to compare the efficacy and safety of lysozyme-based versus benzydamine and chlorhexidine-based oral spray in patients with an acute tonsillopharyngitis associaated with a common cold. Patients and Methods. A prospective two-arm pilot study (lysozyme/cetylpyridinium/lidocaine spray versus: benzydamine spray—arm 1; chlorhexidine/lidocaine spray—arm 2) was conducted in the primary health care unit. Efficacy was evaluated by the patient’s self-assessment of pain, difficulty in swallowing and the throat swelling, by using the visual analog scale (VAS) at baseline and three follow-up visits. Safety was evaluated by the assessment of the frequency and severity of adverse effects. Results. Lysozyme-based spray reduced pain faster than benzydamine-based spray and slower than chlorhexidine-based spray. Lysozyme-based and chlorhexidine-based sprays similarly reduced difficulty in swallowing, but were faster than benzydamine-based spray. Similar effects on the reduction of throat swelling were seen in all treated groups. All tested products showed proper safety and were well tolerated, with no serious adverse events reported. Conclusions. The lysozyme-based oral spray was shown to be effective and safe in the reduction of pain, difficulty in swallowing and throat swelling in patients with acute tonsillopharyngitis associated with a common cold. Lysozyme-based oral spray (containing natural compound with advantages of influencing immune system and preventing recurrences) had similar activity to benzydamine and chlorhexidine-based oral antiseptic sprays.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (1)
Component type: Natural Main substances: Last update: 2019-05-19 20:53:20 | Chemical Risk: |