Diisostearyl malate is an ester derived from malic acid and isostearyl alcohol. It is commonly used in cosmetics as an emollient, providing a smooth, non-greasy feel to formulations. Known for its moisturizing and conditioning properties, it is often included in lipsticks, lip balms, and skincare products to impart a soft, glossy finish. Diisostearyl malate helps improve the texture and spreadability of products, making it an ideal ingredient for formulations that require long-lasting hydration and a silky touch.
Chemical Composition and Structure
Diisostearyl malate is formed through the esterification of malic acid with isostearyl alcohol. Its chemical structure consists of a malate core attached to two isostearyl groups, which are long-chain fatty alcohols. This structure provides excellent emollient properties, allowing it to form a hydrating layer on the skin or lips that locks in moisture.
Physical Properties
Diisostearyl malate typically appears as a clear, colorless to pale yellow liquid with a slightly viscous texture. It is oil-soluble and blends well with other lipophilic ingredients in cosmetic formulations. The ingredient is highly stable and compatible with a wide range of other cosmetic ingredients, and it imparts a soft, smooth texture to formulations without a greasy or heavy feel.
The name describes the structure of the molecule:
- Diisostearyl indicates that the compound consists of two molecules of isostearyl acid bound to malate. Isostearyl acid is a fatty acid obtained from the isomerization of oleic acid and is often used as an emollient and conditioning agent in the production of cosmetics and skincare products.
- Malate indicates the presence of the malate functional group in the molecule. Malate is a salt or ester of malic acid, a carboxylic acid present in nature in many plants and fruits.
Chemical Industrial Synthesis Process
- Selection of Raw Materials. Production begins with the selection of malic acid, an organic acid found in nature, and diisostearyl alcohol, a fatty alcohol primarily derived from plant sources.
- Esterification Reaction. The core of the process is the esterification reaction, where malic acid reacts with diisostearyl alcohol. This reaction requires the use of a catalyst, typically strong acids like sulfuric acid, and heat to accelerate the reaction.
- Reaction Monitoring. Reaction conditions, such as temperature, time, and the ratio of reactants, are carefully monitored to ensure high yield and the formation of the desired ester.
- Purification. After the reaction, the product may contain impurities such as unreacted malic acid, excess alcohol, and catalyst. Purification is carried out using techniques such as vacuum distillation, crystallization, or filtration to obtain a pure final product.
- Quality Control. The purified Diisostearyl Malate undergoes quality control checks to verify its purity, chemical composition, and physical properties, such as melting point and acidity index. These tests can include analytical methods such as high-performance liquid chromatography (HPLC), IR spectroscopy, and titrimetric analyses.
Form and Color
Diisostearyl Malate appears as a colorless or slightly yellow oily liquid.

What it is used for and where
Cosmetics
Skin conditioning agent - Emollient. Emollients have the characteristic of enhancing the skin barrier through a source of exogenous lipids that adhere to the skin, improving barrier properties by filling gaps in intercorneocyte clusters to improve hydration while protecting against inflammation. In practice, they have the ability to create a barrier that prevents transepidermal water loss. Emollients are described as degreasing or refreshing additives that improve the lipid content of the upper layers of the skin by preventing degreasing and drying of the skin. The problem with emollients is that many have a strong lipophilic character and are identified as occlusive ingredients; they are oily and fatty materials that remain on the skin surface and reduce transepidermal water loss. In cosmetics, emollients and moisturisers are often considered synonymous with humectants and occlusives.
Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
Surfactant - Cleansing agent. Cosmetic products used to cleanse the skin utilise the surface-active action that produces a lowering of the surface tension of the stratum corneum, facilitating the removal of dirt and impurities.
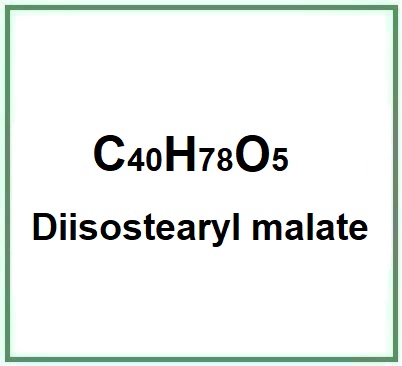
Molecular Formula C40H78O5
Molecular Weight 639.0 g/mol
CAS 67763-18-2 81230-05-9
UNII QBS8A3XZGQ
EC Number 267-041-6
DTXSID30867325
Nikkaji J317.105A
Synonyms
Creaester IM
Haimalate DIS
Pelemol DISM
References_____________________________________________________________________
(1) Hayakawa R, Matsunaga K, Suzuki M, Arima Y, Ohkido Y. Lipstick dermatitis due to C18 aliphatic compounds. Contact Dermatitis. 1987 Apr;16(4):215-9. doi: 10.1111/j.1600-0536.1987.tb01428.x. PMID: 3595121.
Abstract. An 18-year-old girl developed cheilitis. She had a past history of lip cream dermatitis, but the cause was not found. Patch tests with 2 lipsticks were strongly positive. Tests with the ingredients were positive to 2 aliphatic compounds, glyceryl diisostearate and diisostearyl malate. Impurities in the materials were suspected as the cause. Analysis by gas chromatography detected 3 chemicals in glyceryl diisostearate and 1 in diisostearyl malate as impurities. Patch testing with the impurities and glyceryl monoisostearate 0.01% pet in glyceryl diisostearate and isostearyl alcohol 0.25% pet in diisostearyl malate were strongly positive. The characteristics common to the 2 chemicals were liquidity at room temperature, branched C18 aliphatic compound and primary alcohol. Chemicals lacking any of the above 3 features did not react.
![]() Diisostearyl malate
Diisostearyl malate