![]() Calcium benzoate
Calcium benzoate
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
9 pts from Ark90
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Calcium benzoate studies" about Calcium benzoate Review Consensus 10 by Ark90 (12432 pt) | 2023-Mar-29 18:59 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Mota, F. J., Ferreira, I. M., Cunha, S. C., Beatriz, M., & Oliveira, P. P. (2003). Optimisation of extraction procedures for analysis of benzoic and sorbic acids in foodstuffs. Food Chemistry, 82(3), 469-473.
Abstract. Benzoic and sorbic acids are the most commonly used preservatives in foodstuffs. They are usually analysed by RP-HPLC. However, in view of the complexity and diversity of foodstuffs composition, appropriate sample preparation procedures are required for reliable extraction of these preservatives from the matrices. Specific extraction procedures for analysis of jams, table olives, spreadable fats, sauces, fruit juices and wines were optimised. Thus, different types of food matrices were chosen, including those with high sugar content, with high fat content and beverages (with and without alcohol). A significant set of validation data was performed through recovery and precision studies. Chromatographic separation was achieved using a C18 column (S10 ODS2) and acetate buffer 0.005 M (pH=4.4)—methanol (65:35) as mobile phase, 1.4 ml/min flow rate and UV detection at 235 nm. The concentration of preservatives in the samples was calculated by external standard method. Benzoic and sorbic acids in jams, jellies and table olives were efficiently extracted with methanol after ground homogenization. Fortified samples, at 4 different concentration levels of benzoic and sorbic acids, presented average recoveries (after discarding outliers) for each preservative greater than 91% with a coefficient of variation (CV) less than 2.6%. Sorbic acid was extracted from spreadable fats and emulsified sauces with n-hexane and was back-extracted to an aqueous phase with acetate buffer 0.005 M (pH=4.4). Recoveries were higher than 98% for two levels of concentration and CV lower than 2.9%. The preservatives extraction from fruit juices (orange, apple and pineapple) and wines required purification using a Sep-Pak C18 cartridge, and its elution with methanol. Average recoveries of benzoic and sorbic acids at two levels of concentration were greater than 94% with CV less than 4.0%. Eighty-seven commercial brands were analysed including table olives (29), jams (24), jellies (2) spreadable fats (25), sauces (3), fruit juices (10) and table wines (3). All samples conformed to the legal prescriptions.
Terakita A, Byrn SR. Structure and physical stability of hydrates and thermotropic mesophase of calcium benzoate. J Pharm Sci. 2006 May;95(5):1162-72. doi: 10.1002/jps.20589.
Abstract. The aim of this study is to investigate the hydration and the dehydration processes of calcium benzoate hydrates (trihydrate and monohydrate), thermotropic mesophases (dehydrated mesophase and lyophilized mesophase) and amorphous state, and the influence of their molecular order on those processes. X-ray analysis revealed that trihydrate has a planar structure composed of two types of planes-one from benzoic acid, water, and calcium ion and another from benzoic acid and water-and that both planes are linked by three water molecules. It was found that calcium benzoate was able to exist as thermotropic mesophases by dehydration of trihydrate and lyophilization. These mesophases were characterized by polarizing-light microscopy (PLM), X-ray powder diffraction (XRPD), differential thermal analysis (DTA), thermogravimetric analysis (TGA), and scanning electron microscopy (SEM). Both mesophases prepared by two procedures showed some similar physical properties, but lyophilized mesophase seemed to have molecular structure with higher order than dehydrated mesophase. The mesophases exhibited different hydration behavior. The dehydrated mesophase showed a stepwise rehydration process where it became monohydrate first and then trihydrate. The lyophilized mesophase became trihydrate without appearance of monohydrate. An amorphous form could also be prepared and it rehydrated first to the monohydrate and then trihydrate. The results suggest that the more disordered dehydrated mesophase and amorphous state change to monohydrate whereas the more ordered lyophilized mesophase cannot change to monohydrate but only to trihydrate.
Blustein G, Zinola CF. Inhibition of steel corrosion by calcium benzoate adsorption in nitrate solutions: theoretical and experimental approaches. J Colloid Interface Sci. 2004 Oct 15;278(2):393-403. doi: 10.1016/j.jcis.2004.06.009.
Abstract. The inhibitive effects of calcium benzoate on steel corrosion were studied in sodium nitrate solutions at room temperature. Corrosion parameters of the steel/nitrate and steel/benzoate + nitrate interfaces were obtained from polarization curves. Adsorption parameters of benzoate on steel in sodium nitrate solutions were determined through changes in the degree of surface coverage by the inhibitor, as a function of concentration, time, and adsorption potential. The most likely adsorption configuration of benzoate on iron was envisaged with the help of semiempirical calculations such as extended Hückel calculations. A two-dimensional flat configuration was involving at least two metal atoms, one interacting with the phenolic group and the other with the carboxylate moiety. The effect of chloride on the corrosion inhibition of benzoate was analyzed by exposing the metal to different chloride solution concentrations, from which corrosion parameters were calculated and compared with those in nitrate solutions.
Mroz Z, Jongbloed AW, Partanen KH, Vreman K, Kemme PA, Kogut J. The effects of calcium benzoate in diets with or without organic acids on dietary buffering capacity, apparent digestibility, retention of nutrients, and manure characteristics in swine. J Anim Sci. 2000 Oct;78(10):2622-32. doi: 10.2527/2000.78102622x.
Abstract. Eight barrows (Yorkshire x [Finnish Landrace x Dutch Landrace]), initially 30 kg BW, were fitted with ileal cannulas to evaluate the effects of supplementing Ca benzoate (2.4%) and organic acids (OA) in the amount of 300 mEq acid/kg feed on dietary buffering capacity (BC), apparent digestibility and retention of nutrients, and manure characteristics. Swine were allotted in a 2 x 4 factorial arrangement of treatments according to a cyclic (8 x 5) changeover design. Two tapioca-corn-soybean meal-based diets were formulated without and with acidogenic Ca benzoate. Each diet was fed in combination with OA (none, formic, fumaric, or n-butyric acid). Daily rations were equal to 2.8 x maintenance requirement (418 kJ ME/BW(.75)) and were given in two portions. Chromic oxide (.25 g/kg) was used as a marker. On average, Ca benzoate lowered BC by 54 mEq/kg feed. This salt enhanced (P < .05) the ileal digestibility (ID) of DM, OM, arginine, isoleucine, leucine, phenylalanine, alanine, aspartic acid, and tyrosine (by up to 2.4 percentage units). Also, the total tract digestibility (TD) of DM, ash, Ca and GE, and Ca retention (percentage of intake) was greater (P < .05) in swine fed Ca benzoate, whereas N retention remained unaffected. Addition of all OA (formic and n-butyric acid, in particular) exerted a positive effect (P < .05) on the ID of amino acids (except for arginine, methionine, and cysteine). A similar effect (P < .05) was found for the TD of DM, OM, CP, Ca and total P and for the retention of N and Ca. In swine fed Ca benzoate, urinary pH decreased by 1.6 units (P < .001). In conclusion, dietary OA have a beneficial effect on the apparent ileal/total tract nutrient digestibilities, and Ca benzoate increased urine acidity, which could be effective against a rapid ammonia emission from manure of swine.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Calcium benzoate Review Consensus 9 by Ark90 (12432 pt) | 2023-Mar-29 19:02 |
| Read the full Tiiip | (Send your comment) |
Calcium benzoate is a chemical compound, the calcium salt of benzoic acid.
It appears as a white crystalline powder.

What it is used for and where
Cosmetics
It is a restricted ingredient as a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. In particular, all finished products containing substances listed in Annex V/1a that release formaldehyde must be labelled with the warning "contains formaldehyde" if the concentration of formaldehyde in the finished product exceeds 0.05 %.
Preservative. Any product containing organic, inorganic compounds, water, needs to be preserved from microbial contamination. Preservatives act against the development of harmful microorganisms and against oxidation of the product.
Food
Ingredient on the European food additives list as E213, preservative. Calcium benzoate is an agent against bacteria, moulds, yeasts.
Safety
The EFSA Panel on Food Additives considered the use of benzoic acid and its sodium and potassium salts as food additives to be of no concern with regard to genotoxicity and ruled out carcinogenic potential (1).
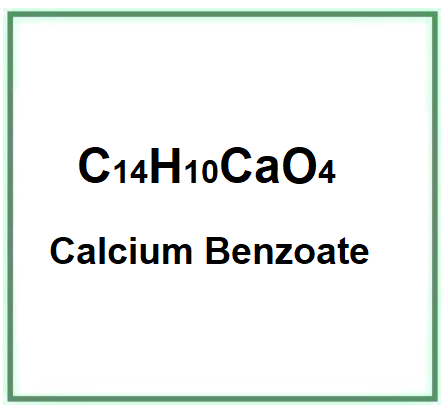
Molecular Formula C14H10CaO4
Molecular Weight 282.30
CAS 2090-05-3 5743-30-6
UNII 3QDE968MKD
EC Number 246-376-1 218-235-4
DSSTox ID DTXSID4044612
IUPAC calcium;dibenzoate
InChl=1S/2C7H6O2.Ca/c2*8-7(9)6-4-2-1-3-5-6;/h2*1-5H,(H,8,9);/q;;+2/p-2
InChl Key NSQPPSOSXWOZNH-UHFFFAOYSA-L
SMILES C1=CC=C(C=C1)C(=O)[O-].C1=CC=C(C=C1)C(=O)[O-].[Ca+2]
MDL number MFCD00050767
NCI C92224
NACRES NA.24
Synonyms:
- Benzoic acid,calcium salt
- Calcium dibenzoate
- calcium(2+) dibenzoate
References_____________________________________________________________________
(1) EFSA Panel on Food Additives and Nutrient Sources (ANS). (2016). Scientific Opinion on the re‐evaluation of benzoic acid (E 210), sodium benzoate (E 211), potassium benzoate (E 212) and calcium benzoate (E 213) as food additives. EFSA Journal, 14(3), 4433.
Abstract. The EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) was asked to deliver a scientific opinion re-evaluating benzoic acid (E 210), sodium benzoate (E 211), potassium benzoate (E 212) and calcium benzoate (E 213) when used as food additives. Benzoic acid and its sodium and potassium salts are rapidly absorbed after oral administration. The Panel considered that the absorption, distribution, metabolism and excretion of calcium benzoate will be similar to sodium or potassium salt and, therefore, read-across between the salts was possible. The results of short-term and subchronic studies on benzoic acid and its salts indicate that their toxicity is low. The Panel considered that the use of benzoic acid and its sodium and potassium salts as food additives does not raise a concern with respect to genotoxicity and, based on read-across, also considered that this conclusion is applicable for calcium benzoate. Moreover, the Panel noted that the available data did not indicate any carcinogenic potential. A four-generation reproductive toxicity study with benzoic acid in the diet in rats was considered by the Panel as the pivotal study and a no observed adverse effect level of 500 mg benzoic acid/kg body weight (bw) per day, the highest dose tested, was identified. From the aforementioned studies, the Panel derived an acceptable daily intake (ADI) of 5 mg/kg bw per day (expressed as benzoic acid) using an uncertainty factor of 100. Taking into account food categories for which direct addition of benzoic acid-benzoates is authorised, the group ADI was exceeded in the brand-loyal scenario in particular for toddlers and children consuming on a regular basis flavoured drinks. Considering additional exposure due to carry-over, the intake could be increased by up to two to three fold for all high-level consumers compared to the previous scenario with only direct addition to food. This results in exceedance of the group ADI in toddlers and children for the non-brand-loyal scenario. The main food categories contributing to this exceedance were unprocessed fruits and vegetables and flavoured drinks.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Main substances: Last update: 2023-03-29 18:17:54 | Chemical Risk: |