Acid Yellow 23 best known as CI 19140 (Yellow 5 or Tartrazine), is a synthetic chemical product, an azo derivative used mainly in the food and cosmetic fields.
CI 19140, commonly known as Tartrazine, is a synthetic food coloring from the azo dye family. It is used in a wide range of food, cosmetic, and pharmaceutical products to impart a bright yellow hue. Tartrazine is one of the most widely used colorants in the industry, employed to color foods, beverages, cosmetics, and medicines. It is highly favored for its stability and the vivid color it provides.
Chemical Composition and Structure
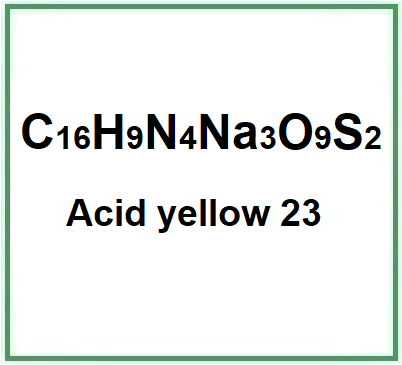
Tartrazine is an azo dye, meaning it contains an azo group (-N=N-) linking two aromatic rings. Its chemical formula is C16H9N4Na3O9S2, and its structure includes sulfonated groups, making it water-soluble. It is a synthetic dye created in laboratories and does not occur naturally in any food or substance.
Physical Properties
CI 19140 appears as a bright yellow powder or granules, soluble in water. It is known for maintaining color stability over time, even when exposed to light or heat. This stability makes it ideal for a wide range of industrial applications.
Production Process
Tartrazine is produced through a chemical process involving the synthesis of organic compounds. The basic ingredients, such as aromatic rings and azo groups, are treated with chemical reagents to form the final dye. This process is carefully controlled to ensure the purity and safety of the product.
Description of the raw materials used in its production.
Aniline. An organic compound used as a starting point in the synthesis of many azo dyes.
Sulfuric and nitric acid. Used for the nitration of aniline.
Hydrochloric acid. Used in the formation of the diazonium salt.
Tartaric acid. Reacts with the diazonium salt to form Tartrazine.
Step-by-step summary of its industrial chemical synthesis process.
- Nitration of aniline using sulfuric and nitric acid to form 4-nitroaniline.
- The 4-nitroaniline is then reduced to 4-aminoaniline.
- The 4-aminoaniline is converted into a diazonium salt using hydrochloric acid and sodium nitrite.
- The diazonium salt reacts with tartaric acid to form Acid Yellow 23 or Tartrazine.
- The product is then purified and isolated for use as a dye.
What it is for and where
It is an ingredient whose primary function is to colour the solution in which it is inserted in a temporary, semi-permanent or permanent manner, either alone or in the presence of complementary components added for colouration.
It occurs in the form of a yellow powder.

Cosmetics
It is a restricted ingredient as IV/44 (CI 19140) III/189 (Acid Yellow 23; Acid Yellow 23 Aluminum lake) a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Substance or ingredient reported: Trisodium 5-hydroxy-1-(4-sulphophenyl)-4-((4-sulphophenyl)azo)pyrazole-3-carboxylate and its insoluble barium, strontium and zirconium lakes, salts and pigments.
Cosmetics - INCI Functions
- Colorant. This ingredient has the function of colouring the solution in which it is inserted in a temporary, semi-permanent or permanent manner, either alone or in the presence of the complementary components added for colouring.
- Hair dyeing. It is an ingredient that adds a colouring to the hair that can be temporary, semi-permanent or permanent depending on what other ingredients are added to achieve the result. The pH for hair dyeing is generally between 9 and 10.
Safety. The problem with azo dyes (monoazo or diazo) is photocatalytic degradation leading to oxidation and subsequent formation of impurities such as aromatic amines, some of which have carcinogenic activity (1).
Health and Safety Considerations
Safety in Use
CI 19140 is considered safe for use in food, cosmetics, and pharmaceuticals within the limits set by regulatory authorities. However, in some people, especially those who are sensitive, it can trigger allergic reactions or intolerances, such as rashes or worsening of asthma. The European Union and other regulatory bodies require that the presence of tartrazine is clearly indicated on product labels.
Allergic Reactions
Some individuals, particularly those with an intolerance to azo dyes, may experience allergic reactions to tartrazine, including itching, hives, or respiratory symptoms. It has also been linked to hyperactivity in children, although scientific evidence on this connection is inconclusive.
Toxicity and Carcinogenicity
There is no conclusive evidence that tartrazine is carcinogenic or toxic when used at approved doses. However, excessive use should be avoided to minimize potential side effects.
Environmental and Safety Considerations
As a synthetic dye, tartrazine is biodegradable, but large quantities can have an environmental impact if not disposed of properly. It is important for industries to follow waste treatment regulations to reduce pollution.
Regulatory Status
CI 19140 is approved for use in many countries, including the European Union, the United States, and Canada, but is subject to restrictions and concentration limits, especially in food products. In some regions, warning labels are required to inform consumers of possible adverse health effects.
CI 19140 studies
- Molecular Formula C16H9N4Na3O9S2
- Molecular Weight 534.36 g/mol
- CAS 1934-21-0
- EC number 217-699-5
Synonyms:
- Tartrazine
- Food Yellow 4
- 1310yellow
References________________________________________________________________________
(1) Chung KT, Stevens SE Jr, Cerniglia CE. The reduction of azo dyes by the intestinal microflora. Crit Rev Microbiol. 1992;18(3):175-90. doi: 10.3109/10408419209114557.
Abstract. Azo dyes are widely used in the textile, printing, paper manufacturing, pharmaceutical, and food industries and also in research laboratories. When these compounds either inadvertently or by design enter the body through ingestion, they are metabolized to aromatic amines by intestinal microorganisms. Reductive enzymes in the liver can also catalyze the reductive cleavage of the azo linkage to produce aromatic amines. However, evidence indicates that the intestinal microbial azoreductase may be more important than the liver enzymes in azo reduction. In this article, we examine the significance of the capacity of intestinal bacteria to reduce azo dyes and the conditions of azo reduction. Many azo dyes, such as Acid Yellow, Amaranth, Azodisalicylate, Chicago Sky Blue, Congo Red, Direct Black 38, Direct Blue 6, Direct Blue 15, Direct Brown 95, Fast Yellow, Lithol Red, Methyl Orange, Methyl Red, Methyl Yellow, Naphthalene Fast Orange 2G, Neoprontosil, New Coccine, Orange II, Phenylazo-2-naphthol, Ponceau 3R, Ponceau SX, Red 2G, Red 10B, Salicylazosulphapyridine, Sunset Yellow, Tartrazine, and Trypan Blue, are included in this article. A wide variety of anaerobic bacteria isolated from caecal or fecal contents from experimental animals and humans have the ability to cleave the azo linkage(s) to produce aromatic amines. Azoreductase(s) catalyze these reactions and have been found to be oxygen sensitive and to require flavins for optimal activity. The azoreductase activity in a variety of intestinal preparations was affected by various dietary factors such as cellulose, proteins, fibers, antibiotics, or supplementation with live cultures of lactobacilli.
![]() Acid Yellow 23
Acid Yellow 23