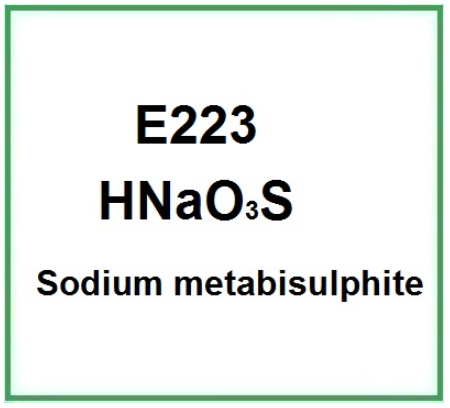
E223 (Sodium metabisulphite) is the sodium salt of sulphurous acid, a chemical compound that belongs to the sulphite group, sulphur-based components that release sulphur dioxide SO2, an active preservative compound.
It appears as a stable white crystalline powder with a slight odour of sulphur dioxide. Soluble in water. Incompatible with strong oxidising agents, strong acids. On contact with strong acids it releases a poisonous gas.

The sulphite group includes:
| Sulphur dioxide | E220 | SO2 |
| Sodium sulphite | E221 | Na2SO3 |
| Sodium hydrogen sulphite | E222 | NaHO3S |
| Sodium metabisulphite | E223 | Na2O5S2 |
| Potassium metabisulphite | E224 | K2O5S2 |
| Calcium sulphite | E226 | CaSO3 |
| Calcium hydrogen sulphite | E227 | CaH2O6S2 |
| Potassium hydrogen sulphite | E228 | KHSO3 |
What it is used for and where
It is a preservative, used by the food industry and labelled with the number E223 on the European Food Additives List.
It is used in foods as a bleaching agent and to prevent browning reactions (1).
Other uses
- In agriculture it is used as a chemical fertiliser, herbicide and pesticide.
- Processing agent for non-ferrous metals, coagulator in rubber processing with functions of bleaching agent, mordant, reducing agent.
- Wastewater treatment agent, water purifier, gas purification agent.
- Oxygen scavenger corrosion inhibitor in the oil and gas industry.
- Reagent for chromatographic analysis.
- Preservative and reducing agent for dyes.
- Dechlorination agent after cotton bleaching and additive for hot cotton processing.
Safety
It has been declared a safe component for human health since 1959 (Gunnison and Jacobsen, 1987; Kencebay et al.2013).
Episode of allergic contact dermatitis caused by a catheter system containing sodium metabisulphite (2).
Symptoms attributable to sulphite sensitivity can be of varying nature and importance. The most common are headache and generalised itching or swelling, but cases of nausea, bronchoconstriction, diarrhoea, hypotension and shock have also occurred (3).
EFSA's Scientific Panel on Food Additives and Flavourings assessed the risk for toxic elements in sulphur dioxide (E 220-228), based on data submitted by stakeholders, and concluded that the EU specification maximum limits for arsenic, lead and mercury should be lowered and a maximum limit for cadmium should be introduced (4).
Sodium metabisulfite studies
Sulphites studies
Caratteristiche tipiche del prodotto commerciale Sodium metabisulfite
| Appearance | White powder |
| pH | 4.0-4.6 |
Melting Point
| 150°C |
| Density | 1.48 |
| PSA | 189.898254 |
| LogP | 0.27220 |
Water Solubility
| 540 g/L (20 ºC) |
Water insoluble
| 0.01% max |
| Fe | 0.002% max |
| As | 0.00006% max |
| Heavy Metals(Pb) | 0.0003% max |
| Safety |  |
- Molecular Formula : Na2O5S2 Na2S2O5
- Molecular Weight : 190.095 g/mol
- Exact Mass 189.898254
- CAS : 7681-57-4 7757-74-6
- UNII 4VON5FNS3C
- EC Number 231-673-0
- DSSTox Substance ID
- IUPAC Sodium metabisulfite
- InChI=1S/2Na.H2O5S2/c;;1-6(2)7(3,4)5/h;;(H,1,2)(H,3,4,5)/q2*+1;/p-2
- InChl Key HRZFUMHJMZEROT-UHFFFAOYSA-L
- SMILES [O-]S(=O)S(=O)(=O)[O-].[Na+].[Na+]
- MDL number MFCD00167602
- PubChem Substance ID 329824616
- ChEBI 114786
- NACRES NA.21
- RXCUI 36696
- RTECS UX8225000
- UN 1759
- ICSC 1461
Synonyms
- Sodium disulfite
- Sodium bisulphite
- Sodium pyrosulfite
- Sodium bisulfite anhydrous
- Natrium pyrosulfit
- Disodium disulfite
- Disodium disulphite
- disodium oxido(oxo)-kappa(4)-sulfanesulfonate
References________________________________________________________________
(1) Dalefield RR, Mueller U. Gastric mucosal irritation following oral exposure to sodium metabisulphite: A reproducible effect? Regul Toxicol Pharmacol. 2016 Oct;80:277-82. doi: 10.1016/j.yrtph.2016.07.005.
(2) Grosch E, Mahler V. Allergic contact dermatitis caused by a catheter system containing sodium metabisulfite. Contact Dermatitis. 2017 Mar;76(3):186-187. doi: 10.1111/cod.12675.
(3) Gunnison AF, Jacobsen DW. Sulfite hypersensitivity. A critical review. CRC Crit Rev Toxicol. 1987;17(3):185-214. doi: 10.3109/10408448709071208.
Abstract. Sulfiting agents (sulfur dioxide and the sodium and potassium salts of bisulfite, sulfite, and metabisulfite) are widely used as preservatives in foods, beverages, and pharmaceuticals. Within the past 5 years, there have been numerous reports of adverse reactions to sulfiting agents. This review presents a comprehensive compilation and discussion of reports describing reactions to ingested, inhaled, and parenterally administered sulfite. Sulfite hypersensitivity is usually, but not exclusively, found within the chronic asthmatic population. Although there is some disagreement on its prevalence, a number of studies have indicated that 5 to 10% of all chronic asthmatics are sulfite hypersensitive. This review also describes respiratory sulfur dioxide sensitivity which essentially all asthmatics experience. Possible mechanisms of sulfite hypersensitivity and sulfur dioxide sensitivity are discussed in detail. Sulfite metabolism and the role of sulfite oxidase in the detoxification of exogenous sulfite are reviewed in relationship to the etiology of sulfite hypersensitivity.
(4) EFSA Panel on Food Additives and Flavourings (FAF); Younes M, Aquilina G, Castle L, Engel KH, Fowler PJ, Frutos Fernandez MJ, Fürst P, Gundert-Remy U, Gürtler R, Husøy T, Manco M, Mennes W, Moldeus P, Passamonti S, Shah R, Waalkens-Berendsen I, Boon P, Cheyns K, Crebelli R, FitzGerald R, Lambré C, Mirat M, Ulbrich B, Vleminckx C, Mech A, Rincon AM, Tard A, Horvath Z, Wright M. Follow-up of the re-evaluation of sulfur dioxide (E 220), sodium sulfite (E 221), sodium bisulfite (E 222), sodium metabisulfite (E 223), potassium metabisulfite (E 224), calcium sulfite (E 226), calcium bisulfite (E 227) and potassium bisulfite (E 228). EFSA J. 2022 Nov 24;20(11):e07594. doi: 10.2903/j.efsa.2022.7594.
Abstract. Sulfur dioxide-sulfites (E 220-228) were re-evaluated in 2016, resulting in the setting of a temporary ADI of 0.7 mg SO2 equivalents/kg bw per day. Following a European Commission call for data, the present follow-up opinion assesses data provided by interested business operators (IBOs) and additional evidence identified in the publicly available literature. No new biological or toxicological data addressing the data gaps described in the re-evaluation were submitted by IBOs. Taking into account data identified from the literature search, the Panel concluded that there was no substantial reduction in the uncertainties previously identified in the re-evaluation. Therefore, the Panel considered that the available toxicity database was inadequate to derive an ADI and withdrew the current temporary group acceptable daily intake (ADI). A margin of exposure (MOE) approach was considered appropriate to assess the risk for these food additives. A lower confidence limit of the benchmark dose of 38 mg SO2 equivalents/kg bw per day, which is lower than the previous reference point of 70 mg SO2 equivalents/kg bw per day, was estimated based on prolonged visual evoked potential latency. An assessment factor of 80 was applied for the assessment of the MoE. At the estimated dietary exposures, when using a refined exposure scenario (Data set D), MOEs at the maximum of 95th percentile ranges were below 80 for all population groups except for adolescents. The dietary exposures estimated using the maximum permitted levels would result in MOEs below 80 in all population groups at the maximum of the ranges of the mean, and for most of the population groups at both minimum and maximum of the ranges at the 95th percentile. The Panel concluded that this raises a safety concern for both dietary exposure scenarios. The Panel also performed a risk assessment for toxic elements present in sulfur dioxide-sulfites (E 220-228), based on data submitted by IBOs, and concluded that the maximum limits in the EU specifications for arsenic, lead and mercury should be lowered and a maximum limit for cadmium should be introduced.
![]() E223
E223