![]() E224
E224
Rating : 5
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Cons:
Specific allergy (1)10 pts from FCS777
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about E224 Review Consensus 10 by FCS777 (5554 pt) | 2023-Dec-08 16:35 |
| Read the full Tiiip | (Send your comment) |
E224 (Potassium metabisulphite) is a chemical compound that belongs to the sulphite group, sulphur-based components that release sulphur dioxide SO2, an active preservative compound, a white granular powder.

E224 is a food additive known as potassium metabisulfite.
The name describes the structure of the molecule:
- The letter E followed by a number is a coding system used in the European Union to identify food additives approved for use in food. These additives are tested for safety and are listed with an "E" code followed by a unique number.
- 224 is the specific identifier for potassium metabisulfite. This compound is used as a preservative and antimicrobial agent in various food products. The E200 series (E200-E297) lists preservatives and a few decolorizers, mostly chemical.
Potassium metabisulphite
The name defines the structure of the molecule
- "Potassium" is an alkali metal, symbol K, with atomic number 19. It's an essential element for life and plays a key role in regulating blood pressure and cellular functions.
- "Metabisulphite" indicates the presence of a metabisulphite anion. It is a food additive and a preservative agent.
Description of raw materials used in production
- Sulfur Dioxide (SO2) - Gas used in the production of sulfites.
- Potassium Hydroxide (KOH) - Strong base used to neutralize the formed sulfurous acid.
Step-by-step summary of industrial chemical synthesis process.
- Production of SO2 - Burning of sulfur produces sulfur dioxide (SO2).
- Reaction with KOH - Sulfur dioxide (SO2) reacts with potassium hydroxide (KOH) to form potassium bisulfite (KHSO3).
- Oxidation - Potassium bisulfite is then oxidized to form potassium metabisulfite (K2S2O5).
- Crystallization - The potassium metabisulfite solution is cooled to allow crystallization.
- Filtration - Crystals are separated from the solution by filtration.
- Drying - Moisture is removed from the crystals.
- Quality Control - The final product undergoes various quality checks to ensure it meets specifications.
The sulphite group includes:
| Sulphur dioxide | E220 | SO2 |
| Sodium sulphite | E221 | Na2SO3 |
| Sodium hydrogen sulphite | E222 | NaHO3S |
| Sodium metabisulphite | E223 | Na2O5S2 |
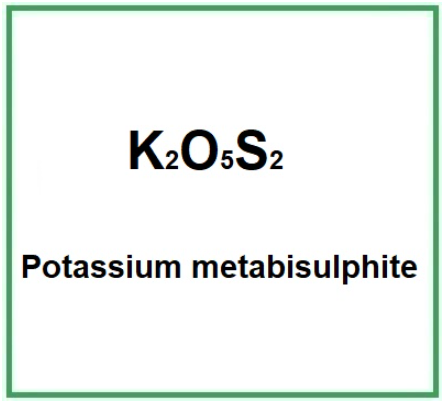
| Potassium metabisulphite | E224 | K2O5S2 |
| Calcium sulphite | E226 | CaSO3 |
| Calcium hydrogen sulphite | E227 | CaH2O6S2 |
| Potassium hydrogen sulphite | E228 | KHSO3 |
What it is used for and where
It is used in the food sector as preservative and antioxidant and is labeled in Europe with the number E224 in food additives.
Can give allergy.
Most significant studies:
It is added to winesas preservative and appears on the label as "Sulfites", in vegetables and dried fruit and dried fruit, mushrooms and in many food products to increase the storage time (1).
It is also used to obtain better sensory qualities to foods (2).
In addition to essential oils, potassium metabisulfite (KMS) may be useful as a repellent for Drosophila suzukii, a globally invasive pest of soft-skinned fruit (3).
Safety
Symptoms related to sulphite sensitivity can be of varying nature and importance. The most common are headache and generalised itching or swelling, but cases of nausea, bronchoconstriction, diarrhoea, hypotension and shock have also occurred (4).
EFSA's Scientific Panel on Food Additives and Flavourings assessed the risk for toxic elements in sulphur dioxide (E 220-228), based on data submitted by stakeholders, and concluded that the EU specification maximum limits for arsenic, lead and mercury should be lowered and a maximum limit for cadmium should be introduced (5).
Sodium Sulfite, Ammonium Sulfite, Sodium Bisulfite, Potassium Bisulfite, Ammonium Bisulfite, Sodium Metabisulfite, and Potassium Metabisulfite are inorganic salts that function as reducing agents in cosmetic formulations. All except Sodium Metabisulfite also function as hair-waving/straightening agents. In addition, Sodium Sulfite, Potassium Sulfite, Sodium Bisulfite, and Sodium Metabisulfite function as antioxidants. Although Ammonium Sulfite is not in current use, the others are widely used in hair care products. Sulfites that enter mammals via ingestion, inhalation, or injection are metabolized by sulfite oxidase to sulfate. In oral-dose animal toxicity studies, hyperplastic changes in the gastric mucosa were the most common findings at high doses. Ammonium Sulfite aerosol had an acute LC(50) of >400 mg/m(3) in guinea pigs. A single exposure to low concentrations of a Sodium Sulfite fine aerosol produced dose-related changes in the lung capacity parameters of guinea pigs. A 3-day exposure of rats to a Sodium Sulfite fine aerosol produced mild pulmonary edema and irritation of the tracheal epithelium. Severe epithelial changes were observed in dogs exposed for 290 days to 1 mg/m(3) of a Sodium Metabisulfite fine aerosol. These fine aerosols contained fine respirable particle sizes that are not found in cosmetic aerosols or pump sprays. None of the cosmetic product types, however, in which these ingredients are used are aerosolized. Sodium Bisulfite (tested at 38%) and Sodium Metabisulfite (undiluted) were not irritants to rabbits following occlusive exposures. Sodium Metabisulfite (tested at 50%) was irritating to guinea pigs following repeated exposure. In rats, Sodium Sulfite heptahydrate at large doses (up to 3.3 g/kg) produced fetal toxicity but not teratogenicity. Sodium Bisulfite, Sodium Metabisulfite, and Potassium Metabisulfite were not teratogenic for mice, rats, hamsters, or rabbits at doses up to 160 mg/kg. Generally, Sodium Sulfite, Sodium Metabisulfite, and Potassium Metabisulfite were negative in mutagenicity studies. Sodium Bisulfite produced both positive and negative results. Clinical oral and ocular-exposure studies reported no adverse effects. Sodium Sulfite was not irritating or sensitizing in clinical tests. These ingredients, however, may produce positive reactions in dermatologic patients under patch test. In evaluating the positive genotoxicity data found with Sodium Bisulfite, the equilibrium chemistry of sulfurous acid, sulfur dioxide, bisulfite, sulfite, and metabisulfite was considered. This information, however, suggests that some bisulfite may have been present in genotoxicity tests involving the other ingredients and vice versa. On that basis, the genotoxicity data did not give a clear, consistent picture. In cosmetics, however, the bisulfite form is used at very low concentrations (0.03% to 0.7%) in most products except wave sets. In wave sets, the pH ranges from 8 to 9 where the sulfite form would predominate. Skin penetration would be low due to the highly charged nature of these particles and any sulfite that did penetrate would be converted to sulfate by the enzyme sulfate oxidase. As used in cosmetics, therefore, these ingredients would not present a genotoxicity risk. The Cosmetic Ingredient Review Expert Panel concluded that Sodium Sulfite, Potassium Sulfite, Ammonium Sulfite, Sodium Bisulfite, Ammonium Bisulfite, Sodium Metabisulfite, and Potassium Metabisulfite are safe as used in cosmetic formulations (6).
Molecular Formula K2S2O5 or K2O5S2
Molecular Weight 222.312 g/mol
UNII 65OE787Q7W
CAS 4429-42-9 16731-55-8
EC Number 240-795-3
Synonyms :
- Potassium metabisulfite
- Dipotassium disulfite
- Dipotassium pyrosulfite
- Dipotassium disulphite
- Dipotassium metabisulfite
- E224
- Potassium disulfite
- Potassium pyrosulfite
- Disulfurous acid, potassium salt (1:2)
- Potassium metabisulfite (NF)
References_________________________________________________________________
(1) Diamante LM, Bai X, Busch J. Fruit Leathers: Method of Preparation and Effect of Different Conditions on Qualities. Int J Food Sci. 2014;2014:139890. doi: 10.1155/2014/139890. Epub 2014 May 4. Review.
Kumar A, Singh M, Singh G. Effect of different pretreatments on the quality of mushrooms during solar drying. J Food Sci Technol. 2013 Feb;50(1):165-70. doi: 10.1007/s13197-011-0320-5.
Sra SK, Sandhu KS, Ahluwalia P. Effect of treatments and packaging on the quality of dried carrot slices during storage. J Food Sci Technol. 2014 Apr;51(4):645-54. doi: 10.1007/s13197-011-0575-x.
(2) Diamante LM, Bai X, Busch J. Fruit Leathers: Method of Preparation and Effect of Different Conditions on Qualities. Int J Food Sci. 2014;2014:139890. doi: 10.1155/2014/139890.
(3) Renkema JM, Wright D, Buitenhuis R, Hallett RH. Plant essential oils and potassium metabisulfite as repellents for Drosophila suzukii (Diptera: Drosophilidae). Sci Rep. 2016 Feb 19;6:21432. doi: 10.1038/srep21432.
(4) Gunnison AF, Jacobsen DW. Sulfite hypersensitivity. A critical review. CRC Crit Rev Toxicol. 1987;17(3):185-214. doi: 10.3109/10408448709071208.
Abstract. Sulfiting agents (sulfur dioxide and the sodium and potassium salts of bisulfite, sulfite, and metabisulfite) are widely used as preservatives in foods, beverages, and pharmaceuticals. Within the past 5 years, there have been numerous reports of adverse reactions to sulfiting agents. This review presents a comprehensive compilation and discussion of reports describing reactions to ingested, inhaled, and parenterally administered sulfite. Sulfite hypersensitivity is usually, but not exclusively, found within the chronic asthmatic population. Although there is some disagreement on its prevalence, a number of studies have indicated that 5 to 10% of all chronic asthmatics are sulfite hypersensitive. This review also describes respiratory sulfur dioxide sensitivity which essentially all asthmatics experience. Possible mechanisms of sulfite hypersensitivity and sulfur dioxide sensitivity are discussed in detail. Sulfite metabolism and the role of sulfite oxidase in the detoxification of exogenous sulfite are reviewed in relationship to the etiology of sulfite hypersensitivity.
(5) EFSA Panel on Food Additives and Flavourings (FAF); Younes M, Aquilina G, Castle L, Engel KH, Fowler PJ, Frutos Fernandez MJ, Fürst P, Gundert-Remy U, Gürtler R, Husøy T, Manco M, Mennes W, Moldeus P, Passamonti S, Shah R, Waalkens-Berendsen I, Boon P, Cheyns K, Crebelli R, FitzGerald R, Lambré C, Mirat M, Ulbrich B, Vleminckx C, Mech A, Rincon AM, Tard A, Horvath Z, Wright M. Follow-up of the re-evaluation of sulfur dioxide (E 220), sodium sulfite (E 221), sodium bisulfite (E 222), sodium metabisulfite (E 223), potassium metabisulfite (E 224), calcium sulfite (E 226), calcium bisulfite (E 227) and potassium bisulfite (E 228). EFSA J. 2022 Nov 24;20(11):e07594. doi: 10.2903/j.efsa.2022.7594.
Abstract. Sulfur dioxide-sulfites (E 220-228) were re-evaluated in 2016, resulting in the setting of a temporary ADI of 0.7 mg SO2 equivalents/kg bw per day. Following a European Commission call for data, the present follow-up opinion assesses data provided by interested business operators (IBOs) and additional evidence identified in the publicly available literature. No new biological or toxicological data addressing the data gaps described in the re-evaluation were submitted by IBOs. Taking into account data identified from the literature search, the Panel concluded that there was no substantial reduction in the uncertainties previously identified in the re-evaluation. Therefore, the Panel considered that the available toxicity database was inadequate to derive an ADI and withdrew the current temporary group acceptable daily intake (ADI). A margin of exposure (MOE) approach was considered appropriate to assess the risk for these food additives. A lower confidence limit of the benchmark dose of 38 mg SO2 equivalents/kg bw per day, which is lower than the previous reference point of 70 mg SO2 equivalents/kg bw per day, was estimated based on prolonged visual evoked potential latency. An assessment factor of 80 was applied for the assessment of the MoE. At the estimated dietary exposures, when using a refined exposure scenario (Data set D), MOEs at the maximum of 95th percentile ranges were below 80 for all population groups except for adolescents. The dietary exposures estimated using the maximum permitted levels would result in MOEs below 80 in all population groups at the maximum of the ranges of the mean, and for most of the population groups at both minimum and maximum of the ranges at the 95th percentile. The Panel concluded that this raises a safety concern for both dietary exposure scenarios. The Panel also performed a risk assessment for toxic elements present in sulfur dioxide-sulfites (E 220-228), based on data submitted by IBOs, and concluded that the maximum limits in the EU specifications for arsenic, lead and mercury should be lowered and a maximum limit for cadmium should be introduced.
(6) Nair B, Elmore AR; Cosmetic Ingredients Review Expert Panel. Final report on the safety assessment of sodium sulfite, potassium sulfite, ammonium sulfite, sodium bisulfite, ammonium bisulfite, sodium metabisulfite and potassium metabisulfite. Int J Toxicol. 2003;22 Suppl 2:63-88.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (1)
Component type: Chemical Main substances: Last update: 2023-08-19 21:38:09 | Chemical Risk: |