Check the ingredients!
... live healthy!


![]() Nisin
Nisin
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Antibacterial (1)10 pts from Whiz35
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Nisin studies" about Nisin Review Consensus 10 by Whiz35 (11828 pt) | 2023-Apr-06 21:21 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Shin, J. M., Gwak, J. W., Kamarajan, P., Fenno, J. C., Rickard, A. H., & Kapila, Y. L. (2016). Biomedical applications of nisin. Journal of applied microbiology, 120(6), 1449-1465.
Abstract. Nisin is a bacteriocin produced by a group of Gram‐positive bacteria that belongs to Lactococcus and Streptococcus species. Nisin is classified as a Type A (I) lantibiotic that is synthesized from mRNA and the translated peptide contains several unusual amino acids due to post‐translational modifications. Over the past few decades, nisin has been used widely as a food biopreservative. Since then, many natural and genetically modified variants of nisin have been identified and studied for their unique antimicrobial properties. Nisin is FDA approved and generally regarded as a safe peptide with recognized potential for clinical use. Over the past two decades the application of nisin has been extended to biomedical fields. Studies have reported that nisin can prevent the growth of drug‐resistant bacterial strains, such as methicillin‐resistant Staphylococcus aureus, Streptococcus pneumoniae, Enterococci and Clostridium difficile. Nisin has now been shown to have antimicrobial activity against both Gram‐positive and Gram‐negative disease‐associated pathogens. Nisin has been reported to have anti‐biofilm properties and can work synergistically in combination with conventional therapeutic drugs. In addition, like host‐defence peptides, nisin may activate the adaptive immune response and have an immunomodulatory role. Increasing evidence indicates that nisin can influence the growth of tumours and exhibit selective cytotoxicity towards cancer cells. Collectively, the application of nisin has advanced beyond its role as a food biopreservative. Thus, this review will describe and compare studies on nisin and provide insight into its future biomedical applications.
Lay, C. L., Dridi, L., Bergeron, M. G., Ouellette, M., & Fliss, I. L. (2016). Nisin is an effective inhibitor of Clostridium difficile vegetative cells and spore germination. Journal of medical microbiology, 65(2), 169-175.
Abstract. Clostridium difficile is the most frequently identified enteric pathogen in patients with nosocomial antibiotic-associated diarrhoea and pseudomembranous colitis. Several clinically isolated C. difficile strains are resistant to antibiotics other than metronidazole and vancomycin. Recently, bacteriocins of lactic acid bacteria have been proposed as an alternative or complementary treatment. The aim of this study was to investigate the inhibitory effect of nisin, a bacteriocin produced by several strains of Lactococcus lactis, against clinical isolates of C. difficile. Nisin Z obtained from culture of L. lactis subsp. lactis biovar. diacetylactis was tested along with commercial nisin A. The effect of nisin A on C. difficile spores was also examined. Nisin A and Z both inhibited the growth of all C. difficile isolates, and MICs were estimated at 6.2 μg ml− 1 for nisin Z and 0.8 μg ml− 1 for nisin A. In addition, C. difficile spores were also susceptible to nisin A (25.6 μg ml− 1), which reduced spore viability by 40–50 %. These results suggested that nisin and hence nisin-producing Lactococcus strains could be used to treat C. difficile-associated diarrhoea.
Breukink, E., van Heusden, H.E., Vollmerhaus, P.J., Swiezewska, E., Brunner, L., Walker, S., Heck, A.J. and de Kruijff, B., 2003. Lipid II is an intrinsic component of the pore induced by nisin in bacterial membranes. Journal of Biological Chemistry, 278(22), pp.19898-19903.
Abstract. The peptidoglycan layers surrounding bacterial membranes are essential for bacterial cell survival and provide an important target for antibiotics. Many antibiotics have mechanisms of action that involve binding to Lipid II, the prenyl chain-linked donor of the peptidoglycan building blocks. One of these antibiotics, the pore-forming peptide nisin uses Lipid II as a receptor molecule to increase its antimicrobial efficacy dramatically. Nisin is the first example of a targeted membrane-permeabilizing peptide antibiotic. However, it was not known whether Lipid II functions only as a receptor to recruit nisin to bacterial membranes, thus increasing its specificity for bacterial cells, or whether it also plays a role in pore formation. We have developed a new method to produce large amounts of Lipid II and variants thereof so that we can address the role of the lipid-linked disaccharide in the activity of nisin. We show here that Lipid II is not only the receptor for nisin but an intrinsic component of the pore formed by nisin, and we present a new model for the pore complex that includes Lipid II.
Cheigh, C. I., & Pyun, Y. R. (2005). Nisin biosynthesis and its properties. Biotechnology letters, 27, 1641-1648.
Abstract. The antimicrobial peptide, nisin, produced by several strains of Lactococcus lactis, which belongs to the Class I bacteriocins called lantibiotics, is a small (3.4 kDa), 34-amino acid, cationic, hydrophobic peptide and has the five characteristic (β-methyl)lanthionine rings formed by significant post-translational modification. A cluster of 11 genes has been involved in the biosynthesis of nisin and are proposed to be transcriptionally arranged as nisA(Z)BTCIP, nisRK, and nisFEG. The biosynthesis of nisin is regulated in a growth-phase-dependent manner including nisin-mediated induction which occurs via NisRK two-component regulatory system. This review outlines some of the more recent developments in the properties, regulation and applications of nisin biosynthesis.
Guiotto, A., Pozzobon, M., Canevari, M., Manganelli, R., Scarin, M., & Veronese, F. M. (2003). PEGylation of the antimicrobial peptide nisin A: problems and perspectives. Il Farmaco, 58(1), 45-50.
Abstract. Nisin is a natural antimicrobial peptide produced by Lactococcus lactis and widely employed as food preservative. Its low solubility in neutral aqueous solutions, its instability at physiological pH and its rapid breakdown by proteolytic enzymes has limited its use for processed foods (processed cheese, milk and derivatives, canned vegetables). The conjugation to poly(ethylene glycol) (PEG) could improve its solubility and protect it towards enzymes present in non optimally processed food. We report the synthesis of a PEG–nisin conjugate, and the microbiology assays against some bacterial cell lines.
Chen, X., Zhang, X., Meng, R., Zhao, Z., Liu, Z., Zhao, X., ... & Guo, N. (2016). Efficacy of a combination of nisin and p-Anisaldehyde against Listeria monocytogenes. Food Control, 66, 100-106.
Abstract. Nisin is used as a food-safe antimicrobial agent and has been widely applied to daily food products to prevent bacterial growth. However, the practical application of nisin is limited. Although nisin inhibits the growth of Listeria monocytogenes, exposure to this agent is also a risk factor for the development of nisin resistance. p-Anisaldehyde (AS) is used in the pharmaceutical industry to manufacture antimicrobial drugs, but few studies have been reported describing the antibacterial activity of AS in the food field. Combining nisin with AS may enhance antimicrobial activity. The synergy observed in vitro was confirmed by checkerboard microdilution method, with the fractional inhibitory concentration index (FICI) values ranging from 0.125 to 0.281. When AS was combined with nisin, a strong synergistic effect was observed using the time-kill and agar-diffusion assays. SEM and LIVE/DEAD BacLight experiment results also suggest that the bactericidal mechanism of the drug combination involves cell wall lysis and membrane damage. We can use this synergy to achieve a stable antimicrobial effect to overcome antimicrobial drug resistance.
Beasley, S. S., & Saris, P. E. (2004). Nisin-producing Lactococcus lactis strains isolated from human milk. Applied and environmental microbiology, 70(8), 5051-5053.
Abstract. Characterization by partial 16S rRNA gene sequencing, ribotyping, and green fluorescent protein-based nisin bioassay revealed that 6 of 20 human milk samples contained nisin-producing Lactococcus lactis bacteria. This suggests that the history of humans consuming nisin is older than the tradition of consuming fermented milk products.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Nisin Review Consensus 10 by Whiz35 (11828 pt) | 2023-Apr-06 21:30 |
| Read the full Tiiip | (Send your comment) |
Nisin is a small cationic peptide, a natural antimicrobial produced by bacteria belonging to the Lactococcus and Streptococcus species, and interacts with the cytoplasmic membrane and its phospholipid components.
Industrially, it appears in the form of a white powder.

What it is used for and where
Medical
It is a bactericide against Salmonella species, Gram-positive bacteria, Clostridium difficile, widely used by the pharmaceutical industry. It is used to control biofilms (1). However, its instability and low solubility in water limits its use.
Food
It is a chemical compound, an ingredient listed on the European food additives list as E234, serving as a preservative particularly in cheeses and canned foods. It is approved by the Food Drug Administration for use as a food additive (GRAS).
 |  |
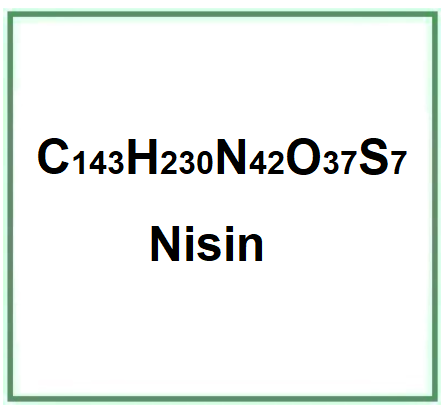
Molecular Formula C143H230N42O37S7
Molecular Weight 3354.07000
CAS 1414-45-5
UNII EN8XKG133D
EC Number 215-807-5
IUPAC (2S)-6-amino-2-[2-[[(2S)-2-[[(2S)-2-[[(2S,3S)-2-[[(2S)-2-[[(1R,4S,7R,11R,14S,17R)-7-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-4-amino-2-[[(3R,9S,12S,15S,21S)-21-[[(2R)-6-amino-2-[[(3R,7R,13S)-3-[[(3R,6S,12S,15R)-15-[[(E)-2-[[(2S,3S)-2-amino-3-methylpentanoyl]amino]but-2-enoyl]amino]-12-[(2S)-butan-2-yl]-9-methylidene-6-(2-methylpropyl)-5,8,11,14-tetraoxo-1-thia-4,7,10,13-tetrazacyclohexadecane-3-carbonyl]amino]-4-methyl-2,9,12-trioxo-5-thia-1,8,11-triazabicyclo[11.3.0]hexadecane-7-carbonyl]amino]hexanoyl]amino]-15,22-dimethyl-12-(2-methylpropyl)-9-(2-methylsulfanylethyl)-5,8,11,14,17,20-hexaoxo-1-thia-4,7,10,13,16,19-hexazacyclodocosane-3-carbonyl]amino]-4-oxobutanoyl]amino]-4-methylsulfanylbutanoyl]amino]hexanoyl]amino]-14-(1H-imidazol-5-ylmethyl)-4,8,20-trimethyl-3,6,12,15,21-pentaoxo-9,19-dithia-2,5,13,16,22-pentazabicyclo[9.9.2]docosane-17-carbonyl]amino]-3-hydroxypropanoyl]amino]-3-methylpentanoyl]amino]-3-(1H-imidazol-5-yl)propanoyl]amino]-3-methylbutanoyl]amino]prop-2-enoylamino]hexanoic acid
InChl=1S/C143H230N42O37S7/c1-24-69(11)105(148)135(213)162-82(27-4)118(196)174-94-58-225-59-95(175-123(201)89(48-67(7)8)169-115(193)74(16)158-138(216)107(70(12)25-2)180-132(94)210)133(211)184-112-79(21)229-61-96(160-104(190)56-152-134(212)100-38-34-44-185(100)142(112)220)128(206)164-84(36-29-32-42-145)120(198)182-109-76(18)226-60-97(161-103(189)55-151-117(195)85(39-45-223-22)165-122(200)88(47-66(5)6)168-113(191)72(14)156-102(188)54-153-136(109)214)129(207)171-92(51-101(147)187)125(203)166-86(40-46-224-23)119(197)163-83(35-28-31-41-144)121(199)183-110-77(19)228-63-99-130(208)170-90(49-80-52-149-64-154-80)124(202)176-98(62-227-78(20)111(141(219)177-99)181-116(194)75(17)159-140(110)218)131(209)173-93(57-186)127(205)179-108(71(13)26-3)139(217)172-91(50-81-53-150-65-155-81)126(204)178-106(68(9)10)137(215)157-73(15)114(192)167-87(143(221)222)37-30-33-43-146/h27,52-53,64-72,75-79,83-100,105-112,186H,15-16,24-26,28-51,54-63,144-146,148H2,1-14,17-23H3,(H2,147,187)(H,149,154)(H,150,155)(H,151,195)(H,152,212)(H,153,214)(H,156,188)(H,157,215)(H,158,216)(H,159,218)(H,160,190)(H,161,189)(H,162,213)(H,163,197)(H,164,206)(H,165,200)(H,166,203)(H,167,192)(H,168,191)(H,169,193)(H,170,208)(H,171,207)(H,172,217)(H,173,209)(H,174,196)(H,175,201)(H,176,202)(H,177,219)(H,178,204)(H,179,205)(H,180,210)(H,181,194)(H,182,198)(H,183,199)(H,184,211)(H,221,222)/b82-27+/t69-,70-,71-,72-,75-,76?,77?,78?,79?,83-,84+,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,105-,106-,107-,108-,109+,110-,111-,112-/m0/s1
InChl Key NVNLLIYOARQCIX-ILAIHEEGSA-N
SMILES CCC(C)C1C(=O)NC(=C)C(=O)NC(C(=O)NC(CSCC(C(=O)N1)NC(=O)C(=CC)NC(=O)C(C(C)CC)N)C(=O)NC2C(SCC(NC(=O)CNC(=O)C3CCCN3C2=O)C(=O)NC(CCCCN)C(=O)NC4C(SCC(NC(=O)CNC(=O)C(NC(=O)C(NC(=O)C(NC(=O)CNC4=O)C)CC(C)C)CCSC)C(=O)NC(CC(=O)N)C(=O)NC(CCSC)C(=O)NC(CCCCN)C(=O)NC5C(SCC6C(=O)NC(C(=O)NC(CSC(C(C(=O)N6)NC(=O)C(NC5=O)C)C)C(=O)NC(CO)C(=O)NC(C(C)CC)C(=O)NC(CC7=CN=CN7)C(=O)NC(C(C)C)C(=O)NC(=C)C(=O)NC(CCCCN)C(=O)O)CC8=CN=CN8)C)C)C)CC(C)C
MDL number MFCD00131724
PubChem Substance ID 24897751
RTECS QU3340000
NACRES NA.85
NSC 112903
Synonyms:
Nisin from Lactococcus lactis
References_____________________________________________________________________
(1) Mahdavi, M., Jalali, M., & Kermanshahi, R. K. (2009). The effect of nisin on biofilm forming foodborne bacteria using microtiter plate method. Research in Pharmaceutical Sciences, 2(2), 113-118.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2023-04-06 19:25:42 | Chemical Risk: |