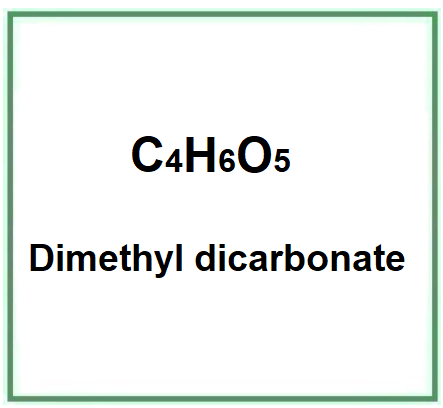
E242 (Dimethyl dicarbonate) is a chemical compound, the dimethyl ester of dicarbonic acid.
It appears as a white powder that is highly reactive and easily hydrolysed in water or as a colourless liquid.

What it is used for and where
Food
Ingredient listed in the European food additives list as E242, preservative. It is used in the preservation of non-alcoholic and alcoholic beverages, especially in wines to prevent yeast spoilage, and is added before the bottling procedure. However, its efficacy in inactivating cellular enzymes is different for different yeast strains and species and is only active on microorganisms that are in a vegetative state (1).
When used in refrigerated environments, Dimethyl dicarbonate tends to solidify below 17° (2).
The critical toxic endpoint (liver) identified in the safety evaluation is carcinogenesis (3).
Dimethyl dicarbonate studies
- Molecular Formula C4H6O5 (CH3OCO)2O
- Molecular Weight 134.09
- CAS 4525-33-1
- UNII 1AY9229ZMG
- EC Number 224-859-8
- DSSTox ID DTXSID2052108
- Nikkaji J205.896K
- IUPAC methoxycarbonyl methyl carbonate
- InChl=1S/C4H6O5/c1-7-3(5)9-4(6)8-2/h1-2H3
- InChl Key GZDFHIJNHHMENY-UHFFFAOYSA-N
- SMILES COC(=O)OC(=O)OC
- MDL number MFCD00008419
- PubChem Substance ID
- ChEBI 173546
- RTECS HT0362500
- Metabolomics Workbench 45763
Synonyms:
- dicarbonic acid dimethyl ester
- methoxycarbonyl methyl carbonate
- Dimethyl pyrocarbonate
References_____________________________________________________________________
(1) Shamsudin, R., Adzahan, N. M., Yee, Y. P., & Mansor, A. (2014). Effect of repetitive ultraviolet irradiation on the physico-chemical properties and microbial stability of pineapple juice. Innovative Food Science & Emerging Technologies, 23, 114-120.
(2) Lück, E., Jager, M., Lück, E., & Jager, M. (1997). Dicarbonic Acid Esters. Antimicrobial Food Additives: Characteristics· Uses· Effects, 168-173.
(3) Kramer NI, Hoffmans Y, Wu S, Thiel A, Thatcher N, Allen TEH, Levorato S, Traussnig H, Schulte S, Boobis A, Rietjens IMCM, Vinken M. Characterizing the coverage of critical effects relevant in the safety evaluation of food additives by AOPs. Arch Toxicol. 2019 Aug;93(8):2115-2125. doi: 10.1007/s00204-019-02501-x.
Abstract. There is considerable interest in adverse outcome pathways (AOPs) as a means of organizing biological and toxicological information to assist in data interpretation and method development. While several chemical sectors have shown considerable progress in applying this approach, this has not been the case in the food sector. In the present study, safety evaluation reports of food additives listed in Annex II of Regulation (EC) No 1333/2008 of the European Union were screened to qualitatively and quantitatively characterize toxicity induced in laboratory animals. The resulting database was used to identify the critical adverse effects used for risk assessment and to investigate whether food additives share common AOPs. Analysis of the database revealed that often such scrutiny of AOPs was not possible or necessary. For 69% of the food additives, the report did not document any adverse effects in studies based on which the safety evaluation was performed. For the remaining 31% of the 326 investigated food additives, critical adverse effects and related points of departure for establishing health-based guidance values could be identified. These mainly involved effects on the liver, kidney, cardiovascular system, lymphatic system, central nervous system and reproductive system. AOPs are available for many of these apical endpoints, albeit to different degrees of maturity. For other adverse outcomes pertinent to food additives, including gastrointestinal irritation and corrosion, AOPs are lacking. Efforts should focus on developing AOPs for these particular endpoints.
![]() E242
E242