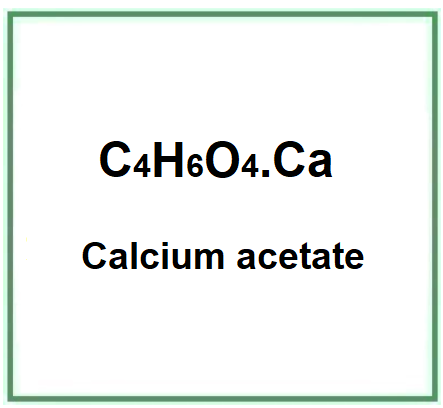
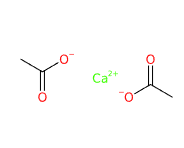
E263 (Calcium acetate) is a chemical compound that is relatively easy and cheap to produce, usually obtained by liquid ion exchange with calcium hydroxide.
It appears as a white powder with an unpleasant taste that is more soluble in cold than in hot water.

What it is used for and where
Medical
Calcium acetate is effective in controlling hyperphosphatemia, a disease that produces elevated levels of phosphate in the blood (1), has been shown to be a potent phosphate binder and has recently been used successfully in chronic dialysis patients (2), and is the most commonly prescribed calcium-containing phosphate binder in the United States.
However, the use of calcium salts to correct hyperphosphatemia has led to progressive coronary and aortic calcification while the use of the non-calcium containing sevelamer binder has not (3) although some studies have raised some doubts (4).
Cosmetics
Fragrance. It plays a decisive and important role in the formulation of cosmetic products as it provides the possibility of enhancing, masking or adding fragrance to the final product, increasing its marketability. The consumer always expects to find a pleasant or distinctive scent in a cosmetic product.
Viscosity control agent. It controls and adapts viscosity to the required level for optimal chemical and physical stability of the product and dosage in gels, suspensions, emulsions, solutions.
Food
Ingredient on the European food additives list as E263, preservative.
Other uses
- organic de-icing salt
- accelerator and water reducer for silica blended cement
The most relevant studies on this ingredient have been selected with a summary of their contents:
"Calcium acetate studies"
- Molecular Formula C4H6O4.Ca (CH3CO2)2Ca
- Molecular Weight 158.17
- CAS 62-54-4
- UNII Y882YXF34X
- EC Number 200-540-9
- DSSTox ID DTXSID0020234
- IUPAC calcium;diacetate
- InChl=1S/2C2H4O2.Ca/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2
- InChl Key VSGNNIFQASZAOI-UHFFFAOYSA-L
- SMILES CC(=O)[O-].CC(=O)[O-].[Ca+2]
- MDL number MFCD00012448
- PubChem Substance ID 329823342
- ChEBI
- RTECS AF7525000
- Nikkaji J4.823B
- FEMA 2228
- ICSC 1092
- NACRES NA.24
Synonyms:
- calcium ethanoate
- calcium(II) acetate
References_____________________________________________________________________
(1) d'Almeida Filho EJ, da Cruz EA, Hoette M, Ruzany F, Keen LN, Lugon JR. Calcium acetate versus calcium carbonate in the control of hyperphosphatemia in hemodialysis patients. Sao Paulo Med J. 2000 Nov 9;118(6):179-84. doi: 10.1590/s1516-31802000000600006.
(2) Pflanz, S., Henderson, I. S., McElduff, N., & Jones, M. C. (1994). Calcium acetate versus calcium carbonate as phosphate-binding agents in chronic haemodialysis. Nephrology Dialysis Transplantation, 9(8), 1121-1124.
(3) Chertow, G.M., Raggi, P., McCarthy, J.T., Schulman, G., Silberzweig, J., Kuhlik, A., Goodman, W.G., Boulay, A., Burke, S.K. and Toto, R.D., 2003. The effects of sevelamer and calcium acetate on proxies of atherosclerotic and arteriosclerotic vascular disease in hemodialysis patients. American journal of nephrology, 23(5), pp.307-314.
(4) Yusuf, A. A., Weinhandl, E. D., & Peter, W. L. S. (2014). Comparative effectiveness of calcium acetate and sevelamer on clinical outcomes in elderly hemodialysis patients enrolled in Medicare part D. American journal of kidney diseases, 64(1), 95-103.
![]() E263
E263