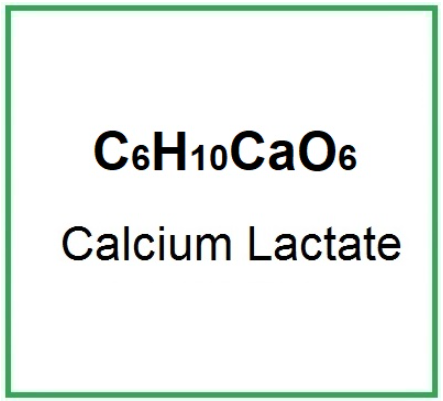
E327 (Calcium lactate) is the calcium salt of lactic acid.
Industrially it appears in the form of a white powder.

What it is used for and where
Food
Acidity corrector, anti-acid and to improve taste.
In pre-cooked foods, particularly hamburgers, it serves to increase shelf life.
Ingredient on the European food additives list as E327, preservative.
Medical
It is used as an adjuvant in calcium deficiencies. In dentistry, it acts as a tooth enamel protector (1).
It is also sold as a dietary supplement to improve athletic performance, but extensive studies have shown its inconsistency (2).
Cosmetics
Cosmetic astringent. This ingredient exerts a direct effect on the skin by tightening dilated pores by contracting stratum corneum cells and removing superfluous oil.
Buffering agent. It is an iingredient that can bring an alkaline or acid solution to a certain pH level and prevent it from changing, in practice a pH stabiliser that can effectively resist instability and pH change.
Keratolytic. It is a lysis agent, i.e. a process of physically removing corneocytes or surface cells on the stratum corneum. It basically removes dead cells.
Molecular Formula: C6H10CaO6 C6H10CaO6 · 5H2O
Molecular Weight: 218.218 g/mol
CAS: 814-80-2
EC Number: 212-406-7
Synonyms:
- calcium 2-hydroxypropanoate
- Calphosan
- Conclyte calcium
- Calcium dilactate
- Hemicalcium L-lactate
- Calcium 2-hydroxypropanoate (1:2)
- 2-Hydroxypropanoic acid calcium salt
- calcium bis(2-hydroxypropanoate)
- Propanoic acid, 2-hydroxy-, calcium salt (2:1)
- Lactic acid, calcium salt (2:1)
References________________________________________________________________________
(1) Calcium lactate pre-rinse increased fluoride protection against enamel erosion in a randomized controlled in situ trial.
Turssi CP, Hara AT, Amaral FL, França FM, Basting RT.
J Dent. 2014 May;42(5):534-9. doi: 10.1016/j.jdent.2014.02.012.
(2) Effect of magnesium lactate dihydrate and calcium lactate monohydrate on 20-km cycling time trial performance.
Peveler WW, Palmer TG.
J Strength Cond Res. 2012 Apr;26(4):1149-53. doi: 10.1519/JSC.0b013e31822dcd7f.
![]() E327
E327