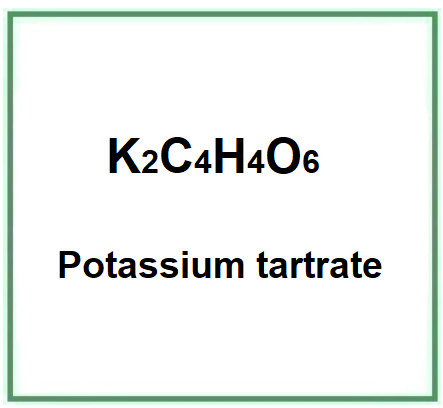
Potassium tartrate is a chemical compound that is the potassium salt of tartaric acid.
The name describes the structure of the molecule.
- Potassium. An essential mineral for the human body, involved in various cellular functions, including regulating heartbeat and muscle function.
- Tartrate. A salt or ester of tartaric acid, an organic acid found in nature, e.g., in grapes.
Description of raw materials used in production.
- The primary raw material for producing potassium tartrate is tartar, a sediment that naturally forms during the fermentation of wine.
Step-by-step summary of industrial production process.
- Tartar collection.
- Purification. The tartar is purified to remove impurities and foreign substances.
- Reaction with potassium hydroxide. The purified tartar is reacted with potassium hydroxide to produce potassium tartrate.
- Crystallization. The solution is then cooled to allow the formation of potassium tartrate crystals.
- Collection and drying. The crystals are collected, washed, and dried.
Form and color. Potassium tartrate appears as white or colorless crystalline powder.

What it is used for and where
Food
Ingredient listed in the European food additives list as E336, acidity regulator.
Food Additive. Used as a stabilizer, pH regulator, and leavening agent in food and beverages.
Baking Products. Often used in baking products as a leavening agent, especially in combination with baking soda.
Wine. Used in the wine-making process to stabilize tartrate crystals.
It is also known as cream of tartar when used in cooking, particularly in the preparation of cakes and baked goods. It is soluble in water and has a slightly acidic taste. As a salt of tartaric acid, potassium tartarate has a variety of uses in the food and other industries, taking advantage of its stabilizing, pH-regulating and leavening properties. It is also a key component in some homemade wine-making kits, where it helps control crystal formation during the fermentation and aging process.
Pharmaceuticals
May be used in the manufacturing of pharmaceutical products as a stabilizing agent and for other purposes.
Cosmetics
Buffering agent. It is an iingredient that can bring an alkaline or acid solution to a certain pH level and prevent it from changing, in practice a pH stabiliser that can effectively resist instability and pH change.
Safety
Tartaric acid (E 334), sodium tartrates (E 335), potassium tartrates (E 336), sodium potassium tartrate (E 337) and calcium tartrate (E 354) are authorised as food additives according to Regulation (EC) No 1333/2008 on food additives. The Panel established a group ADI for l(+)‐tartaric acid and tartrates (E 334-337 and E 354) of 240 mg/kg bw per day, expressed as tartaric acid, by applying the total uncertainty factor of 10 to the reference point of 3,100 mg sodium tartrate/kg bw per day (the highest dose tested), that is approximately 2,440 mg tartaric acid/kg bw per day. Accordingly, the current ADI of 30 mg/kg bw per day is withdrawn (1).
 |  |
- Molecular Formula C4H4K2O6
- Molecular Weight 226.27
- CAS 921-53-9
- UNII O9WLL1ZL8S
- EC Number 203-490-6
- DSSTox ID DTXSID90889442
- IUPAC dipotassium;(2R,3R)-2,3-dihydroxybutanedioate
- InChl=1S/C4H6O6.2K/c5-1(3(7)8)2(6)4(9)10;;/h1-2,5-6H,(H,7,8)(H,9,10);;/q;2*+1/p-2/t1-,2-;;/m1../s1
- InChl Key AVTYONGGKAJVTE-OLXYHTOASA-L
- SMILES C(C(C(=O)[O-])O)(C(=O)[O-])O.[K+].[K+]
- MDL number MFCD00013065
- ChEBI 63018
- Nikkaji J7.170F
Synonyms:
- PotassiumD-tartratemonobasic
- Dipotassium tartrate
- Potassium tartrates
References_____________________________________________________________________
(1) EFSA Panel on Food Additives and Flavourings (FAF); Younes M, Aquilina G, Castle L, Engel KH, Fowler P, Frutos Fernandez MJ, Fürst P, Gürtler R, Gundert-Remy U, Husøy T, Mennes W, Shah R, Waalkens-Berendsen I, Wölfle D, Boon P, Tobback P, Wright M, Aguilera J, Rincon AM, Tard A, Moldeus P. Re-evaluation of l(+)-tartaric acid (E 334), sodium tartrates (E 335), potassium tartrates (E 336), potassium sodium tartrate (E 337) and calcium tartrate (E 354) as food additives. EFSA J. 2020 Mar 11;18(3):e06030. doi: 10.2903/j.efsa.2020.6030.
![]() Potassium tartrate
Potassium tartrate