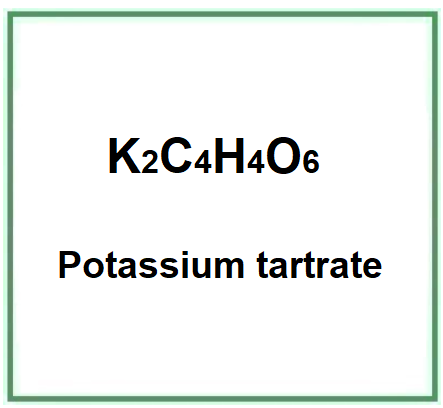
E336 (Potassium tartrate) is a chemical compound, the potassium salt of tartaric acid.
The name describes the structure of the molecule:
- Potassium refers to the potassium ion (K+), an alkaline metal.
- Tartrate is the tartrate ion, which is derived from tartaric acid, a type of organic acid. Tartaric acid has the chemical formula C4H6O6 and, when it loses two hydrogen ions (H+), it becomes a tartrate ion (C4H4O6-2).
The synthesis process takes place in different steps:
- Preparation. Aqueous solutions of tartaric acid, Rochelle salt (potassium sodium tartrate) and potassium sulphate.
- Reaction. Tartaric acid (H2C4H4O6) reacts with Rochelle salt (KNaC4H4O6) and potassium sulphate (K2SO4) to form potassium tartrate (K2C4H4O6), sodium sulphate (Na2SO4) and water (H2O).
- Precipitation. Potassium tartrate precipitates from the solution as it is less soluble in water than the other products.
- Filtration. The solution is filtered to separate the solid potassium tartrate from the liquid.
- Drying. Potassium tartrate is dried to obtain the solid potassium tartrate from the liquid.
It is not metabolized in the body and is eliminated in the urine without any side effects, however an Admissible Daily Dose of 30mg/kg body weight is indicated (1).
It appears in the form of a white powder.

What it is used for and where
Food
Ingredient listed in the European food additives list as E336, acidity regulator.
Cosmetics
Buffering agent. It is an iingredient that can bring an alkaline or acid solution to a certain pH level and prevent it from changing, in practice a pH stabiliser that can effectively resist instability and pH change.
Safety
Tartaric acid (E 334), sodium tartrates (E 335), potassium tartrates (E 336), sodium potassium tartrate (E 337) and calcium tartrate (E 354) are authorised as food additives according to Regulation (EC) No 1333/2008 on food additives. The Panel established a group ADI for l(+)‐tartaric acid and tartrates (E 334-337 and E 354) of 240 mg/kg bw per day, expressed as tartaric acid, by applying the total uncertainty factor of 10 to the reference point of 3,100 mg sodium tartrate/kg bw per day (the highest dose tested), that is approximately 2,440 mg tartaric acid/kg bw per day. Accordingly, the current ADI of 30 mg/kg bw per day is withdrawn (1).
 |  |
- Molecular Formula C4H4K2O6
- Molecular Weight 226.27 g/mol
- CAS 921-53-9
- UNII O9WLL1ZL8S
- EC Number 213-067-8
- DSSTox ID DTXSID90889442
- IUPAC dipotassium;(2R,3R)-2,3-dihydroxybutanedioate
- InChl=1S/C4H6O6.2K/c5-1(3(7)8)2(6)4(9)10;;/h1-2,5-6H,(H,7,8)(H,9,10);;/q;2*+1/p-2/t1-,2-;;/m1../s1
- InChl Key AVTYONGGKAJVTE-OLXYHTOASA-L
- SMILES C(C(C(=O)[O-])O)(C(=O)[O-])O.[K+].[K+]
- MDL number MFCD00013065
- ChEBI 63018
- Nikkaji J7.170F
Synonyms:
- PotassiumD-tartratemonobasic
- Dipotassium tartrate
References_____________________________________________________________________
(1) EFSA Panel on Food Additives and Flavourings (FAF); Younes M, Aquilina G, Castle L, Engel KH, Fowler P, Frutos Fernandez MJ, Fürst P, Gürtler R, Gundert-Remy U, Husøy T, Mennes W, Shah R, Waalkens-Berendsen I, Wölfle D, Boon P, Tobback P, Wright M, Aguilera J, Rincon AM, Tard A, Moldeus P. Re-evaluation of l(+)-tartaric acid (E 334), sodium tartrates (E 335), potassium tartrates (E 336), potassium sodium tartrate (E 337) and calcium tartrate (E 354) as food additives. EFSA J. 2020 Mar 11;18(3):e06030. doi: 10.2903/j.efsa.2020.6030.
![]() E336
E336