![]() E341
E341
Rating : 6
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
10 pts from RS232
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about E341 Review Consensus 10 by RS232 (2058 pt) | 2023-Jun-28 17:45 |
| Read the full Tiiip | (Send your comment) |
E341 (Calcium phosphate) is a chemical compound, calcium salt of phosphoric acid.
The name defines the structure of the molecule:
- Calcium is a chemical element with the symbol Ca and the atomic number 20. It is a soft, gray alkaline metal, and is the fifth most abundant element by mass in the Earth’s crust.
- Phosphate refers to the phosphate ion (PO4 3-), a polyatomic ion with a charge of -3. Phosphates are important in biochemistry for their role in storing and transferring energy within cells.
The synthesis process takes place in several stages:
- Reactive sources of calcium and phosphate. In the first phase a reaction is expected between a source of calcium, such as calcium chloride and a source of phosphate, such as phosphoric acid or ammonium phosphate. This reaction produces calcium phosphate and a by-product, which could be ammonium chloride or water, depending on the phosphate source used.
- Precipitation. Calcium phosphate is precipitated out of the solution by adjusting the pH of the solution, often with a base such as sodium hydroxide. Calcium phosphate precipitates from the solution as a solid.
- Filtration and washing. The precipitated calcium phosphate is filtered from the solution and washed to remove any remaining impurities.
- Drying and grinding. The filtered calcium phosphate is dried and ground into a fine powder ready for use.
It appears as an odourless, tasteless, white powder that is stable in air. Insoluble in water and alcohol, it is easily soluble in dilute hydrochloric and nitric acids.

What it is used for and where
Food
It is used in food in a variety of applications and is labelled as E341 in the European food additives list as an acidity regulator.
- table salt
- seasonings
- food supplements
- bakery products
with antioxidant functions to prevent browning and is also used as a leavening agent.
As a food additive it is distinguished into:
- E341 (i) Calcium dihydrogen phosphate
- E341 (ii) Calcium hydrogen phosphate
- E341 (iii) Tricalcium phosphate
Medical
In medical applications it is used in bone treatment (1), in odontostomatology to protect tooth enamel (2) and to treat granulomas or periapical lesions (3) and to correct intrabony periodontal filling defects (4).
Another interesting technique is the subchondral injection of calcium phosphate, which consists of the application of a synthetic calcium-phosphate bone substitute into the bone marrow with the aim of improving the structural quality of the subchondral bone in order to achieve a modification of the bone to prevent the progression of arthritis and eventual bone collapse (5).
This article reviews the classification of calcinosis, a rheumatic disorder, with the mechanisms that may contribute to the pathogenesis of calcinosis and summarises the evidence evaluating non-pharmacological and pharmacological interventions for the treatment of calcinosis involving hydroxyapatite and calcium phosphate (6).
Bones are an important area of application for biomaterials and grafts to achieve healing from critical size defects are composed of calcium phosphates.
Safety
The Panel on Food Additives and Flavourings added to Food (FAF) provided a scientific opinion re-evaluating the safety of phosphates (E 338–341, E 343, E 450–452) as food additives. The Panel considered that (1). The Panel considered that phosphates have low acute oral toxicity and there is no concern regarding genotoxicity and carcinogenicity. The Panel calculated a group Acceptable Daily Intake (ADI) for phosphates expressed as phosphorus of 40 mg/kg body weight (bw) per day and concluded that this ADI is protective for the human population (7).
Animal nutrition
It increases body weight, reproduction rate and survival rate, shortens the fattening period and improves the defence against diseases such as chondropathy, dysentery and paralysis.
Cosmetics
Abrasive agent. It contains abrasive particles to remove stains or biofilm that accumulate on the stratum corneum or teeth. Baking soda, kieselguhr, silica and many others have abrasive properties. Peeling or exfoliating products used in dermatology or cosmetic applications contain abrasive agents in the form of synthetic microspheres, however these microspheres or abrasive particles are not biodegradable and create pollution in aquatic ecosystems.
Antiplaque agent. This ingredient has the property of preventing the onset of caries by fighting the bacteria responsible for acid corrosion of teeth.
Buffering agent. It is an iingredient that can bring an alkaline or acid solution to a certain pH level and prevent it from changing, in practice a pH stabiliser that can effectively resist instability and pH change.
Bulking agent. It regulates the water content, dilutes other solids, can increase the volume of a product for better flow, acts as a buffer against organic acids, helps to keep the pH of the mixture within a certain level.
Oral care agent. This ingredient can be placed in the oral cavity to improve and/or maintain oral hygiene and health, to prevent or improve a disorder of the teeth, gums, mucous membrane.
Viscosity control agent. It controls and adapts viscosity to the required level for optimal chemical and physical stability of the product and dosage in gels, suspensions, emulsions, solutions.
The most relevant studies on this ingredient have been selected with a summary of their contents:
Typical commercial product characteristics Calcium phosphate
| Appearance | White powder |
| Boiling Point | 158ºC at 760 mmHg |
| Density | 2.306(16℃) |
| Boiling Point | 158ºC a 760 mmHg |
| Water | ≤1.0% |
| Loss on drying | ≤2.0% |
| Sulphated ash | ≤0.5%/1g |
| Residue on ignition | ≤0.1% |
| Heavy metals | ≤10 ppm |
| Storage | Light-resistant and airtight containers. |
 |  |
 |  |
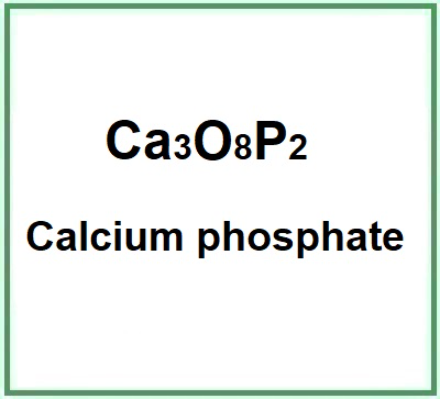
- Molecular Formula CaHO4P Ca3O8P2
- Molecular Weight 136.06
- Exact Mass 135.923843
- CAS 7757-93-9
- UNII K4C08XP666
- EC Number 231-826-1
- DSSTox Substance ID DTXSID1049803
- IUPAC tricalcium;diphosphate
- InChI=1S/3Ca.2H3O4P/c;;;2*1-5(2,3)4/h;;;2*(H3,1,2,3,4)/q3*+2;;/p-6
- InChl Key QORWJWZARLRLPR-UHFFFAOYSA-H
- SMILES [O-]P(=O)([O-])[O-].[O-]P(=O)([O-])[O-].[Ca+2].[Ca+2].[Ca+2]
- MDL number MFCD00010909
- PubChem Substance ID 24892950
- ChEBI 9679
- FEMA 3081
- NACRES NA.25
- RTECS
Synonyms
- Dicalcium phosphate
- Monohydrogen calcium phosphate
- dibasic calcium phosphate
- Calcium hydrogen phosphate
- Calcium phosphate, dibasic
- calcium,hydrogen phosphate
References____________________________________________________________
(1) Kawata M, Azuma K, Izawa H, Morimoto M, Saimoto H, Ifuku S. Biomineralization of calcium phosphate crystals on chitin nanofiber hydrogel for bone regeneration material. Carbohydr Polym. 2016 Jan 20;136:964-9. doi: 10.1016/j.carbpol.2015.10.009.
(2) Shen P, Bagheri R, Walker GD, Yuan Y, Stanton DP, Reynolds C, Reynolds EC. Effect of calcium phosphate addition to fluoride containing dental varnishes on enamel demineralization. Aust Dent J. 2016 Sep;61(3):357-65. doi: 10.1111/adj.12385.
(3) Mostafa AA, Zaazou MH, Chow LC, Mahmoud AA, Zaki DY, Basha M, Abdel Hamid MA, Khallaf ME, Sharaf NF, Hamdy TM. Injectable nanoamorphous calcium phosphate based in situ gel systems for the treatment of periapical lesions. Biomed Mater. 2015 Nov 6;10(6):065006. doi: 10.1088/1748-6041/10/6/065006.
(4) Stevanović M, Biočanin V, Nedkić M, Ignjatović N. Efficacy of nanocrystalline bone substitute biphasic calcium phosphate/poly-DL-lactide-co-glycolide for periodontal intrabony defects filling. Vojnosanit Pregl. 2015 Aug;72(8):689-95. doi: 10.2298/vsp140528049s.
(5) Winge MI, Reikerås O, Røkkum M. Calcium phosphate bone cement: a possible alternative to autologous bone graft. A radiological and biomechanical comparison in rat tibial bone. Arch Orthop Trauma Surg. 2011 Aug;131(8):1035-41. doi: 10.1007/s00402-011-1271-z.
(6) Elahmar H, Feldman BM, Johnson SR. Management of calcinosis cutis in rheumatic diseases. J Rheumatol. 2022 May 15:jrheum.211393. doi: 10.3899/jrheum.211393.
(7) EFSA Panel on Food Additives and Flavourings (FAF); Younes M, Aquilina G, Castle L, Engel KH, Fowler P, Frutos Fernandez MJ, Fürst P, Gürtler R, Husøy T, Mennes W, Moldeus P, Oskarsson A, Shah R, Waalkens-Berendsen I, Wölfle D, Aggett P, Cupisti A, Fortes C, Kuhnle G, Lillegaard IT, Scotter M, Giarola A, Rincon A, Tard A, Gundert-Remy U. Re-evaluation of phosphoric acid-phosphates - di-, tri- and polyphosphates (E 338-341, E 343, E 450-452) as food additives and the safety of proposed extension of use. EFSA J. 2019 Jun 12;17(6):e05674. doi: 10.2903/j.efsa.2019.5674.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (1)
Component type: Chemical Main substances: Last update: 2023-04-12 18:19:11 | Chemical Risk: |