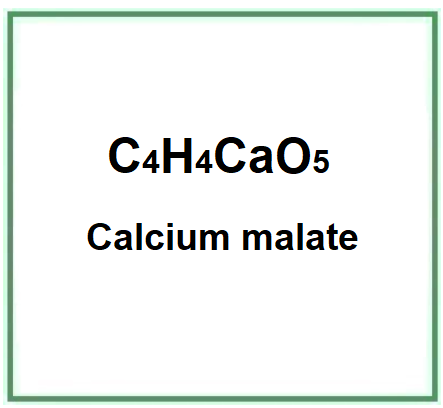
Calcium malate is a chemical compound, the calcium salt of malic acid.
The name defines the structure of the molecule
- Calcium is an alkaline earth metal, symbol Ca, with atomic number 20. It's an essential element for life, crucial for the formation and maintenance of bones and teeth.
- Malate is derived from malic acid, an organic acid found in many plants, especially apples. When malic acid loses two protons (H+), it becomes a dianion, called malate.
Description of raw materials used in production
- Malic Acid is a naturally occurring organic acid that is found in various fruits, especially apples. It serves as the primary precursor for the synthesis of Calcium malate.
- Calcium Hydroxide is used to neutralize the malic acid, resulting in the formation of Calcium malate.
Step-by-step summary of its industrial chemical synthesis process.
Preparation of Malic Acid Solution. Malic acid is dissolved in water to prepare a malic acid solution.
Addition of Calcium Hydroxide. Calcium hydroxide is slowly added to the malic acid solution. This results in a neutralization reaction where malic acid reacts with calcium hydroxide to form Calcium malate.
Crystallization. The solution is allowed to cool, leading to the crystallization of Calcium malate.
Filtration: The crystallized Calcium malate is separated from the solution using filtration.
Drying. The filtered Calcium malate crystals are dried to remove any remaining moisture.
Purification. Depending on the desired purity, further purification steps like recrystallization might be employed.
It appears as a white powder, slightly soluble in water, insoluble in alcohol.

What it is used for and where
Food
Ingredient listed in the European food additives list as E352, buffering agent. Its bitter taste is generally removed by ion exchange.
It is added in unfortified wines and champagnes.
It is subdivided into
- E352 (i) Monocalcium malate
- E352 (ii) Calcium malate
Medical
Source of bioavailable calcium. to improve bone strength without increasing the risk of kidney stones
Other uses
It is one of the most marketable feed additives used in aquaculture.
Safety
In 2007, EFSA's Panel on Food Additives concluded that the use of calcium citrate malate as a source of calcium for use in foods for particular nutritional uses (PARNUTS) and in foods for the general population (including food supplements) does not raise safety concerns at the maximum levels estimated in this opinion (1).
- Molecular Formula C4H4CaO5
- Molecular Weight 172.15
- CAS 17482-42-7
- UNII D48OP746DW
- EC Number 241-498-1
- DSSTox ID DTXSID30936929
- IUPAC calcium;2-hydroxybutanedioate
- InChl=1S/C4H6O5.Ca/c5-2(4(8)9)1-3(6)7;/h2,5H,1H2,(H,6,7)(H,8,9);/q;+2/p-2
- InChl Key OLOZVPHKXALCRI-UHFFFAOYSA-L
- SMILES C(C(C(=O)[O-])O)C(=O)[O-].[Ca+2]
- MDL number MFCD00043793
- Nikkaji J248.901E
Synonyms:
- calcium hydrogenmalate
- calcium 2-hydroxysuccinate
References_____________________________________________________________________
(1) EFSA Panel on Food Additives and Nutrient Sources added to Food (EFSA ANS Panel), Younes, M., Aggett, P., Aguilar, F., Crebelli, R., Dusemund, B., Filipič, M., Frutos, M.J., Galtier, P., Gundert‐Remy, U. and Kuhnle, G.G., 2018. Evaluation of di‐calcium malate, used as a novel food ingredient and as a source of calcium in foods for the general population, food supplements, total diet replacement for weight control and food for special medical purposes. EFSA Journal, 16(6), p.e05291.
![]() Calcium malate
Calcium malate