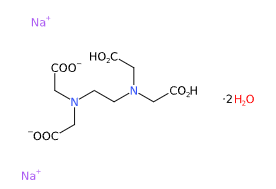
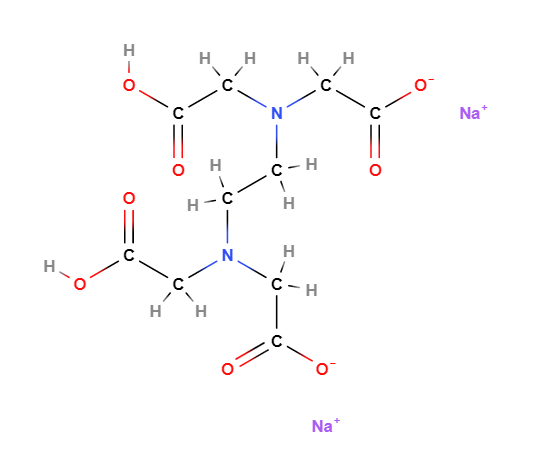
E385 or Disodium EDTA (Ethylenediaminetetraacetic acid disodium salt) is a chemical compound, disodium salt of EDTA, a chelator of calcium ions and, among EDTA salts, the most important.
It appears in the form of a white, odourless, tasteless powder, easily soluble in water, ammonia, aqueous solutions of sodium hydroxide, sodium carbonate, and hardly soluble in organic solvents such as diethyl ether or alcohol.

What it is used for and where
In chemistry, it is a six-atom complexation agent that regulates the equilibrium between ligand and metal ions, often added to lysate with magnesium and calcium complex ions to stabilise nucleic acid and degrade nuclease activity. It controls the polymerisation rate. It also functions as a chelating agent, i.e. an agent that binds to another to promote elimination or harmful reactions caused by metal ions and to form a stable water-soluble complex. These studies explain the mechanism (1). It can chelate with a large majority of different metal ions other than alkali metals such as calcium, magnesium, iron, copper and other polyvalent ions.
It also works as a descaler (2) and as an antioxidant method also called the Fenton method (3).
Widely used to prevent oxidation caused by metals.
Food
Labelled with the number E385 in the list of European food additives, Disodium EDTA is a chemical compound that is added to food as a preservative and chelating agent to prevent spoilage of the product in which it is included and to counteract other ingredients.
It is found in carbonated drinks, jellies, canned beans, mayonnaise and others.
To check for its presence in food, a convenient HPLC method was developed for the quantitative determination of EDTA in food. EDTA in food samples was easily extracted with water using ultrasound. After conversion to Fe (III) complex in the presence of Fe (III) ions, EDTA was separated on a reversed-phase C30 column and detected by ultraviolet detection (260 nm). Citrate and malate, which are present in many foods, also formed Fe (III) complexes but did not interfere with the chromatographic detection of EDTA. The method allowed the determination of EDTA in food at concentrations down to 0.01 mmol/kg. Good recoveries (95.2-101%) were obtained with the standard addition method on four samples with high repeatability (RSD, 0.8-3.4%). The method was successfully applied to the analysis of EDTA in carbonated drinks, jellies, canned beans, canned maize and food supplements (4).
Disodium EDTA is a known enhancer of iron absorption, yet the results of this study suggest that in the presence of EDTA, iron absorption occurs mainly from the paracellular rather than the regulated cellular mode, which could potentially increase its toxicity (5).
There is a maximum ADI (Acceptable Daily Intake) or GDA (Acceptable Daily Allowance) of 2.5 grams per kg body weight for this food additive (6).
Cosmetics
It acts as a chelating agent and pH adjuster in cosmetic formulations. Chelating agents are known to be cytotoxic and weakly genotoxic, but not carcinogenic as confirmed by the final report of the Expert Panel for the Review of Cosmetic Ingredients (7).
Chelating agent. It has the function of preventing unstable reactions and improving the bioavailability of chemical components within a product, and removes calcium and magnesium cations that can cause cloudiness in clear liquids.
Viscosity control agent. It controls and adapts viscosity to the required level for optimal chemical and physical stability of the product and dosage in gels, suspensions, emulsions, solutions.
Medical
Disodium EDTA is used in the form of intravenous administration in chelation therapy for lead and heavy metal poisoning, in the treatment of calcified tendonitis, as an anticoagulant and bactericide due to its low cost.
Other uses
- additive, bleaching and fixing agent for processing colour-sensitive materials
- water purifier and cleaner
- agricultural chemicals
- plant growth inhibitor
- chemical plating, cyanide-free electroplating
For more information:
Disodium EDTA studies
Typical commercial product characteristics Ethylenediaminetetraacetic acid disodium salt
| Appearance | White powder |
| pH | 4.0-5.0 |
| Boiling Point | >100°C 614.2°C at 760 mmHg |
| Melting Point | 248°C |
Flash Point
| 325.2ºC |
| Density | 1.01 g/mL at 25°C |
| Vapor Pressure | 1.15E-16mmHg at 25°C |
| Chlorides | 0.01% Max |
| Sulphates | 0.1% Max |
| Iron | 0.001% Max |
| Heavy metal | 0.001% Max |
| Chelating value | 221 |
| NTA | 0.2% |
| Water insoluble matter | ≤0.005% |
Loss on drying
| 8.7- 11.4% |
Aminotriacetic acid
| ≤10 ppm |
| Storage | 2-8°C |
| Chemical Risk |  |
- Molecular Formula C10H18N2Na2O10 C10H14N2O8Na2.2H2O
- Linear Formula [-CH2N(CH2CO2Na)CH2CO2H]2
- Molecular Weight 336.21
- Exact Mass 336.054565
- CAS 139-33-3 6831-92-6 (Ethylenediaminetetraacetic acid disodium salt dihydrate)
- UNII
- EC Number 205-358-3
- DSSTox Substance ID DTXSID9027073
- IUPAC disodium;2-[2-[carboxylatomethyl(carboxymethyl)amino]ethyl-(carboxymethyl)amino]acetate
- InChl=1S/C10H16N2O8.2Na/c13-7(14)3-11(4-8(15)16)1-2-12(5-9(17)18)6-10(19)20;;/h1-6H2,(H,13,14)(H,15,16)(H,17,18)(H,19,20);;/q;2*+1/p-2
- InChl Key ZGTMUACCHSMWAC-UHFFFAOYSA-L
- SMILES C(CN(CC(=O)O)CC(=O)[O-])N(CC(=O)O)CC(=O)[O-].[Na+].[Na+]
- MDL number MFCD00070672
- PubChem Substance ID 329747771
- RTECS AH4410000
- NCI C65508
- FEMA 4520
- HS Code 2922499990
- Beilstein 3822669
- eCl@ss 39030907
- NACRES NA.25
Synonyms :
Ethylenediaminetetraacetic acid, Disodium salt, Disodic EDTA, Disodic edetate, Acid Edetic
References_____________________________________________________________________
(1) Retrievability of calcium hydroxide intracanal medicament with three calcium chelators, ethylenediaminetetraacetic acid, citric acid, and chitosan from root canals: An in vitro cone beam computed tomography volumetric analysis.
Raghu R, Pradeep G, Shetty A, Gautham PM, Puneetha PG, Reddy TVS.
J Conserv Dent. 2017 Jan-Feb;20(1):25-29. doi: 10.4103/0972-0707.209068.
Comparison of efficiency of ethylenediaminetetraacetic acid, citric acid, and etidronate in the removal of calcium hydroxide intracanal medicament using scanning electron microscopic analysis: An in-vitro study.
Chockattu SJ, Deepak BS, Goud KM.
J Conserv Dent. 2017 Jan-Feb;20(1):6-11. doi: 10.4103/0972-0707.209079.
(2) An in vitro study on the efficacy of removing calcium hydroxide from curved root canal systems in root canal therapy.
Wang Y, Guo LY, Fang HZ, Zou WL, Yang YM, Gao Y, Yang H, Hu T.
Int J Oral Sci. 2017 Jun;9(2):110-116. doi: 10.1038/ijos.2017.14. Epub 2017 Jun 23.
(3) Cosmeceutical Effects of Galactomannan Fraction from Arenga pinnata Fruits In vitro.
Yanti, Madriena, Ali S.
Pharmacognosy Res. 2017 Jan-Mar;9(1):39-45. doi: 10.4103/0974-8490.199773.
Effect of Selected Plant Phenolics on Fe2+-EDTA-H₂O₂ System Mediated Deoxyribose Oxidation: Molecular Structure-Derived Relationships of Anti- and Pro-Oxidant Actions.
de Graft-Johnson J, Nowak D.
Molecules. 2016 Dec 31;22(1). pii: E59. doi: 10.3390/molecules22010059.
(4) Determination of ethylenediaminetetraacetic acid in foods by reversed-phase high-performance liquid chromatography.
Kemmei T, Kodama S, Yamamoto A, Inoue Y, Hayakawa K.
Food Chem. 2013 Jun 1;138(2-3):866-9. doi: 10.1016/j.foodchem.2012.11.103. Epub 2012 Dec 5.
(5) Interactions between ethylenediaminetetraacetic acid (EDTA) and iron absorption pathways, in the Caco-2 model.
Kibangou IB, Bureau F, Allouche S, Arhan P, Bouglé D.
Food Chem Toxicol. 2008 Nov;46(11):3414-6. doi: 10.1016/j.fct.2008.08.014. Epub 2008 Aug 20.
(6) http://www.fao.org/ag/agn/jecfa-additives/specs/Monograph1/Additive-164.pdf
(7) Lanigan RS, Yamarik TA. Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA. Int J Toxicol. 2002;21 Suppl 2:95-142. doi: 10.1080/10915810290096522.
![]() E385
E385