E536 (Potassium ferrocyanide) is a chemical compound obtained by the reaction of sodium ferrocyanide with calcium hydroxide and potassium chloride. It is a non-toxic cyanide.
It appears as a whitish or yellowish crystalline powder.

What it is used for and where
Food
Ingredient included in the list of European food additives as E536 with an anti-caking function in particular to prevent agglomeration of table salt.
Safety
EFSA's Panel on Food Additives provided a scientific opinion reviewing the safety of sodium ferrocyanide (E535), potassium ferrocyanide (E536) and evaluating the safety of calcium ferrocyanide (E538) as food additives. Exposure to ferrocyanides (E535-538) from their use as food additives was up to 0.009 mg/kg body weight (bw) per day in children and adolescents. In the refined estimated exposure scenario, the exposure was up to 0.003 mg/kg bw per day in children and adolescents. Absorption of ferrocyanides is low and there is no accumulation in humans. The Panel established a group Acceptable Daily Intake (ADI) for sodium, potassium and calcium ferrocyanide of 0.03 mg/kg bw per day expressed as ferrocyanide ion. The Panel concluded that ferrocyanides (E535-538) are of no safety concern at current authorised levels of use (1).
Subsequent to this 2018 report, in 2019 a group of Dutch researchers conducted a study on the 'Pathways of Adverse Outcomes and Risk Assessment' of 326 food additives of which the majority are emulsifiers and stabilisers (87), acidity regulators and antioxidants (58). Some food additives were found to be nephrotoxic, including sodium, potassium and calcium ferrocyanide (E535, E536 and E538) (2).
Medical
Dentistry. Potassium ferrocyanide has been used in the treatment of dental caries (3).
Other uses
- effective in lowering the voltage applied for electrochromism (4)
- removes excessive amounts of heavy metals in wines (5)
- pigment production
- titration of zinc in the presence of other interfering metals
- probing of electron transfer reactions in solid electrodes.
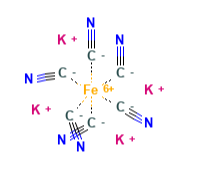
- Molecular Formula K4[Fe(CN)6] · 3H2O
- Molecular Weight 422.4 g/mol
- CAS 13943‐58‐3 anhydrous 14459‐95‐1 hydrate
- UNII
- EC Number 237‐722‐2
References_____________________________________________________________________
(1) EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipič M, Frutos MJ, Galtier P, Gott D, Gundert-Remy U, Kuhnle GG, Lambré C, Leblanc JC, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Waalkens-Berendsen I, Wright M, Di Domenico A, Van Loveren H, Giarola A, Horvath Z, Lodi F, Woutersen RA. Re-evaluation of sodium ferrocyanide (E 535), potassium ferrocyanide (E 536) and calcium ferrocyanide (E 538) as food additives. EFSA J. 2018 Jul 25;16(7):e05374. doi: 10.2903/j.efsa.2018.5374.
(2) Kramer NI, Hoffmans Y, Wu S, Thiel A, Thatcher N, Allen TEH, Levorato S, Traussnig H, Schulte S, Boobis A, Rietjens IMCM, Vinken M. Characterizing the coverage of critical effects relevant in the safety evaluation of food additives by AOPs. Arch Toxicol. 2019 Aug;93(8):2115-2125. doi: 10.1007/s00204-019-02501-x.
(3) Anderson, R.W. and Knutson, J.W., 1951. Effect of topically applied zinc chloride and potassium ferrocyanide on dental caries experience. Public Health Reports (1896-1970), pp.1064-1066.
(4) Mori, H. and Mizuguchi, J., 1987. Green electrochromism in the system of p-cyanophenylviologen and potassium ferrocyanide. Japanese journal of applied physics, 26(8R), p.1356.
(5) Edge, R.A., 1958. The detection of cyanides and ferro-cyanides in wines. South African Journal of Agricultural Science, 1(3), p.337.


![]() E536
E536