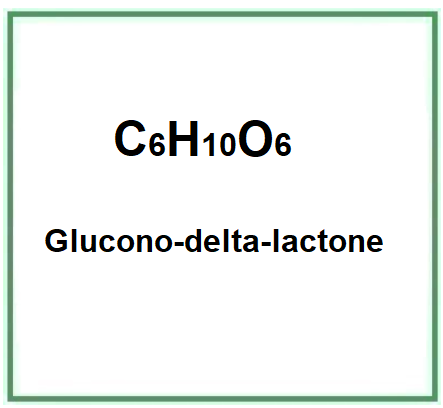
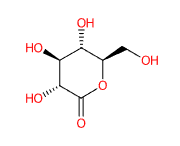
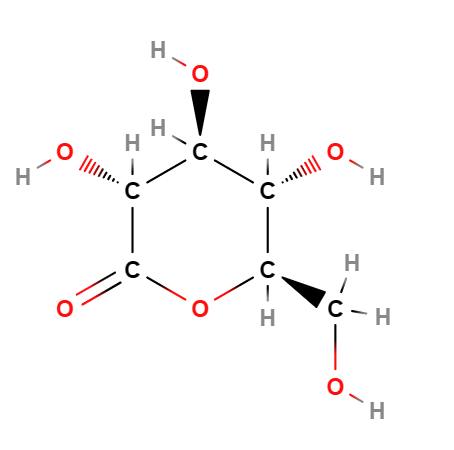
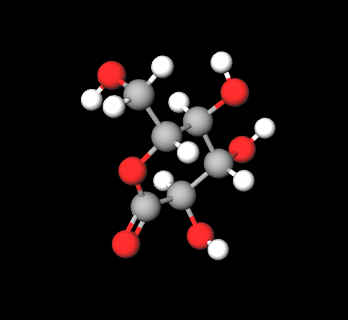
E575 (Glucono-delta-lactone) is a a cyclical ester, natural polyhydroxy acid produced by enzymatic oxidation from D-glucose and has a scavenging capacity in free radicals with a strong antioxidant action. It has also demonstrated hydrating and chelating activity and is commonly used in cosmetics, medicine and in the food industry as an acidifying, chelating, leavening or, as in the case of tofu processing, coagulating agent.
The name describes the structure of the molecule. "Glucono" refers to the gluconic acid from which it is derived. " Lactone" refers to its cyclic ester form.
The synthesis process takes place in several stages:
- Step 1: Preparation of raw materials: gluconic acid, which can be obtained by fermentation of glucose with the fungus Aspergillus niger.
- Step 2: Dehydration. Gluconic acid is dehydrated to form glucone delta-lactone by heating gluconic acid in the presence of a dehydrating agent.
- Step 3: Purification. The resulting Gluconolactone is then purified to remove any unreacted gluconic acid and by-products by various filtration, distillation and crystallization techniques.
- Step 4: Drying. The purified Gluconolactone is then dried to remove any residual solvent.
It comes in white or brown powder form.

What it is used for and where
Food
Ingredient included in the list of European food additives as E575 as acidifier and acidity regulator
Medical
It has demonstrated ability to dissolve stones by irrigation.
It has long been used, as a replacement for benzoyl peroxide, in the treatment of acne because it affects keratinization and reduces inflammatory lesions. It also has fewer side effects than benzoyl peroxide (1).

The results of this study demonstrate the ability of gluconolactone PHA to protect against UV-induced activation of the elastin promoter. In addition, in vivo studies have shown that gluconolactone treatment does not cause a significant increase in sunburn cells. Further investigation of this and other PHAs is needed to identify their potential role in the prevention and repair of skin photodamage (2).
In some laboratory animal studies, changes in behavioral activity have been shown in animals treated with gluconolactone and neurotoxicity may be hinted at when consumed in higher than acceptable doses. These changes have been shown to be elevated in combination with taurine (3).
Cosmetics
- Chelating agent. It has the function of preventing unstable reactions and improving the bioavailability of chemical components within a product, and removes calcium and magnesium cations that can cause cloudiness in clear liquids.
- Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
Gluconolattone studies
- Molecular Formula: C6H10O6
- Molecular Weight: 178.14 g/mol
- CAS: 90-80-2
- UNII WQ29KQ9POT
- EC Number: 202-016-5
- DSSTox Substance ID: DTXSID0026549
- MDL number MFCD00006647
- PubChem Substance ID 24895089
- Beilstein/REAXYS Number: 83286
- NACRES: NA.21
Synonyms:
- delta-gluconolactone
1,5-Gluconolactone
D-glucono-1,5-lactone
(3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-one
References_________________________________________________________________
(1) Hunt MJ, Barnetson RS. A comparative study of gluconolactone versus benzoyl peroxide in the treatment of acne. Australas J Dermatol. 1992;33(3):131-4. doi: 10.1111/j.1440-0960.1992.tb00100.x.
(2) Bernstein EF, Brown DB, Schwartz MD, Kaidbey K, Ksenzenko SM. The polyhydroxy acid gluconolactone protects against ultraviolet radiation in an in vitro model of cutaneous photoaging. Dermatol Surg. 2004 Feb;30(2 Pt 1):189-95; discussion 196. doi: 10.1111/j.1524-4725.2004.30060.x.
(3) Boyina R, Dodoala S. Evaluation of the Neurobehavioural Toxic Effects of Taurine, Glucuronolactone, and Gluconolactone Used in Energy Drinks in Young Rats. Turk J Pharm Sci. 2020 Dec 23;17(6):659-666. doi: 10.4274/tjps.galenos.2019.33602.
![]() E575
E575