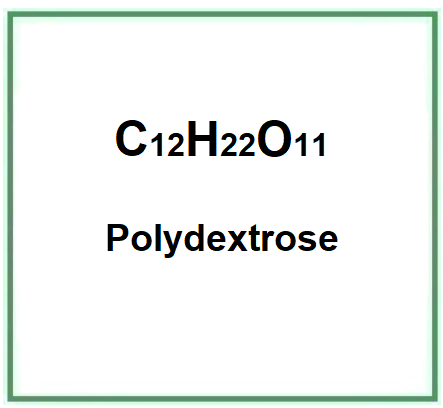
E1200 (Polydextrose) is a carbohydrate, oligosaccharide, glucose polymer with a high molecular weight, containing small amounts of sorbitol and citric acid. Industrially it is produced by melting and condensation of the ingredients (90 parts D-glucose, 10 parts sorbitol, 1 part citric acid or 0.1 parts phosphoric acid).
It appears as a white, water-soluble powder.

What it is used for and where
Food
Ingredient listed in the European food additives list as E1200 with a stabilising, stabilising and partially preservative function.
It provides similar physiological effects to other dietary fibres and has shown prebiotic potential and to selectively stimulate the growth and/or activity of one or a limited number of gut bacteria associated with different physiological health benefits (1).
Safety
EFSA's Scientific Panel on Food Additives and Flavourings considers that there is no need for a numerical acceptable daily intake (ADI) for polydextrose (E 1200) and that there are no concerns about the safety of reported uses and use levels of polydextrose as a food additive (2).
Cosmetics
Bulking agent. It regulates the water content, dilutes other solids, can increase the volume of a product for better flow, acts as a buffer against organic acids, helps to keep the pH of the mixture within a certain level.
Humectant. Hygroscopic compound used to minimise water loss in the skin and to prevent it from drying out by facilitating faster and greater absorption of water into the stratum corneum of the epidermis. The epidermis is the most superficial of the three layers that make up human skin (epidermis, dermis and hypodermis) and is the layer that maintains hydration in all three layers. In turn, the epidermis is composed of five layers: horny, the most superficial, granular, spinous, shiny, and basal. Humectants have the ability to retain the water they attract from the air in the stratum corneum and have the function of moisturising the skin. They are best used before emollients, which are oil-based.
Polydextrose studies
- Molecular Formula C12H22O11
- Molecular Weight 342.297 g/mol
- CAS 68424-04-4 200-075-1
- UNII VH2XOU12IE
- EC number 614-467-9
Synonyms:
- E1200
- O762
- AS-15284
- MolPort-023-220-455
References______________________________________________________________________
(1) do Carmo MM, Walker JC, Novello D, Caselato VM, Sgarbieri VC, Ouwehand AC, Andreollo NA, Hiane PA, Dos Santos EF. Polydextrose: Physiological Function, and Effects on Health. Nutrients. 2016 Sep 8;8(9):553. doi: 10.3390/nu8090553.
Abstract. Polydextrose (PDX) is a non-digestible oligosaccharide used widely across most sectors of the food industry. It is a randomly linked glucose oligomer containing small amounts of sorbitol and citric acid. The random bonds in PDX prevent mammalian digestive enzymes from readily hydrolyzing the molecule and it has a reported energy value of 1 kcal/g. These properties have led to the acceptance in many countries that PDX provides similar physiological effects as other dietary fibers and has shown prebiotic potential. Dietary intervention with prebiotics has been shown to selectively stimulate the growth and/or activity of one or a limited number of intestinal bacteria associated with several physiological benefits on health. Therefore, the objective of this review was a survey of the literature on the effect of supplementation with PDX in health, and to list the benefits for maintaining health and/or reducing the development of diseases.
(2) EFSA Panel on Food Additives and Flavourings (FAF), Younes, M., Aquilina, G., Castle, L., Engel, K.H., Fowler, P., Fürst, P., Gürtler, R., Gundert‐Remy, U., Husøy, T. and Manco, M., 2021. Re‐evaluation of polydextrose (E 1200) as a food additive. EFSA Journal, 19(1), p.e06363.
Abstract. This opinion deals with the re-evaluation of polydextrose (E 1200) when used as a food additive. The Panel followed the conceptual framework for the risk assessment of certain additives and considered that: adequate exposure estimates were available; the margin of safety (MOS)/margin of exposure (MOE) for arsenic was between 0.5-14 and 8.5 for lead; the exhaustions of the tolerable weekly intake (TWI) for cadmium would be 165%, 10% for mercury, whereas the exhaustion of the tolerable daily intake (TDI) for nickel would be 9%; the absorption is limited and part of polydextrose is fermented in the large intestine into short-chain fatty acids (SCFA); adequate toxicity data were available; there is no concern with respect to genotoxicity; no adverse effects were reported in subchronic studies in rats, dogs or monkeys nor in chronic or carcinogenicity studies in mice and rats at the highest doses tested of up 12,500 mg/kg body weight (bw) per day and 15,000 mg/kg bw per day, respectively; the nephrocalcinosis in dogs given high doses of polydextrose was considered to be a treatment-related but a secondary effect related to diarrhoea, and hence not relevant for the risk assessment; no adverse effects were reported in reproductive or developmental toxicity studies in rats administered up to 10,000 mg polydextrose/kg bw per day, or in a developmental toxicity study in rabbits up to 1,818 mg/kg bw per day (the highest dose tested). Therefore, the Panel concluded that there is no need for numerical acceptable daily intake (ADI) for polydextrose (E 1200), and that there is no safety concern for the reported uses and use levels of polydextrose as a food additive. The Panel recommended that European Commission considers to lower the maximum limit for lead and to introduce limits for arsenic, cadmium and mercury in the EU specifications for polydextrose (E 1200), and to verify that polydextrose-N as a food additive (E 1200) is no longer marketed in the EU. © 2021 European Food Safety Authority. EFSA Journal published by John Wiley and Sons Ltd on behalf of European Food Safety Authority.
![]() E1200
E1200