![]() Phenylephrine hydrochloride
Phenylephrine hydrochloride
Rating : 5
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Cons:
Possible eye irritant (1)10 pts from Frank123
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Phenylephrine Hydrochloride studies" about Phenylephrine hydrochloride Review Consensus 10 by Frank123 (12058 pt) | 2023-May-03 11:39 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Meyer SM, Fraunfelder FT. 3. Phenylephrine hydrochloride. Ophthalmology. 1980 Nov;87(11):1177-80. doi: 10.1016/s0161-6420(80)35108-7.
Abstract. Phenylephrine hydrochloride is a potent, effective, relatively safe drug with few ocular side effects. Side effects from topical instillation are uncommon but include severe systemic cardiovascular effects with elevated blood pressure and stroke. Ten percent phenylephrine should be used with caution in patients with known cardiac disease, hypertension, aneurysms, long-standing insulin-dependent diabetes, or advanced arteriosclerosis. A 2.5% concentration is generally indicated for ophthalmic examination as well as for use in infants and in the elderly. Phenylephrine should not be used in patients with narrow-angle glaucoma, and it is also contraindicated in patients taking monoamine oxidase inhibitors or tricyclic antidepressants.
Esteve-Taboada JJ, Del Águila-Carrasco AJ, Bernal-Molina P, Ferrer-Blasco T, López-Gil N, Montés-Micó R. Effect of Phenylephrine on the Accommodative System. J Ophthalmol. 2016;2016:7968918. doi: 10.1155/2016/7968918.
Abstract. Accommodation is controlled by the action of the ciliary muscle and mediated primarily by parasympathetic input through postganglionic fibers that originate from neurons in the ciliary and pterygopalatine ganglia. During accommodation the pupil constricts to increase the depth of focus of the eye and improve retinal image quality. Researchers have traditionally faced the challenge of measuring the accommodative properties of the eye through a small pupil and thus have relied on pharmacological agents to dilate the pupil. Achieving pupil dilation (mydriasis) without affecting the accommodative ability of the eye (cycloplegia) could be useful in many clinical and research contexts. Phenylephrine hydrochloride (PHCl) is a sympathomimetic agent that is used clinically to dilate the pupil. Nevertheless, first investigations suggested some loss of functional accommodation in the human eye after PHCl instillation. Subsequent studies, based on different measurement procedures, obtained contradictory conclusions, causing therefore an unexpected controversy that has been spread almost to the present days. This manuscript reviews and summarizes the main research studies that have been performed to analyze the effect of PHCl on the accommodative system and provides clear conclusions that could help clinicians know the real effects of PHCl on the accommodative system of the human eye.
Stavert B, McGuinness MB, Harper CA, Guymer RH, Finger RP. Cardiovascular Adverse Effects of Phenylephrine Eyedrops: A Systematic Review and Meta-analysis. JAMA Ophthalmol. 2015 Jun;133(6):647-52. doi: 10.1001/jamaophthalmol.2015.0325.
Abstract. Importance: Topical phenylephrine hydrochloride is routinely administered with few safety precautions, but evidence regarding its systemic safety to date is controversial. As even short-term variations in 24-hour blood pressure (BP) and heart rate (HR) can adversely affect cardiovascular health, better evidence on phenylephrine's effects on HR and BP is required. Objective: To perform a meta-analysis of available evidence regarding cardiovascular adverse effects of topical phenylephrine. Data sources: PubMed, MEDLINE, and the Cochrane Database of Systematic Reviews and Clinical Trials were searched for relevant literature from January 1, 1970, to January 1, 2014, using a combination of the following search terms: topical, ocular, ophthalmic, phenylephrine, tropicamide, cardiovascular effect, side effect, blood pressure, heart rate, mydriatic, and eye drops. A total of 70 articles related to the topic were identified and all full texts were retrieved. Study selection: Randomized clinical trials reporting change in BP and HR for adults were included in this review. All studies reporting results for neonates or infants, not reporting standard deviations, or not specifying the time of measurement or the concentration of phenylephrine used were excluded. Data extraction and synthesis: Data from randomized clinical trials that reported BP and/or HR as well as the time following administration of topical phenylephrine at which measurements were obtained by concentration of phenylephrine as a mean change and its standard deviation were extracted. Data were synthesized by concentration of phenylephrine and time of measurement following topical application using random-effects models with inverse variance weighting to account for heterogeneity across studies. Main outcomes and measures: Difference in BP and HR after topical administration of phenylephrine. Results: Eight RCTs with a total of 916 participants were included. Data were available for phenylephrine, 2.5%, at 20 to 30 minutes and 60 minutes or longer after administration, and neither BP nor HR changed at either time. Following application of phenylephrine, 10%, BP increased at 5 and 10 minutes (mean difference for both, +15 mm Hg; 95% CI, 11.94-18.54; P < .001) but decreased at 20 to 30 minutes and 60 minutes or longer with no changes detected against baseline. A mean increase in HR by 4.48 beats/min (95% CI, 1.09-7.88; P = .01) was present at 20 to 30 minutes following application of phenylephrine, 10%, and HR decreased by 60 minutes or longer with no changes detected compared with baseline. Conclusions and relevance: Phenylephrine, 2.5%, leads to no clinically relevant change in BP or HR, and the changes in BP and HR seen with phenylephrine, 10%, are short lived. Thus, phenylephrine, 2.5%, is safe to use in clinical routine.
Moreno-Ancillo A, Munoz-Robles ML, Cabañas R, Barranco P, Lopez-Serrano MC. Allergic contact reactions due to phenylephrine hydrochloride in eyedrops. Ann Allergy Asthma Immunol. 1997 Jun;78(6):569-72. doi: 10.1016/s1081-1206(10)63217-6.
Abstract. Background: Dermatitis of and around the eye is common. Allergic contact reactions from phenylephrine are rare despite extensive use by ophthalmologists. Previous reports do not indicate crossreactivity between phenylephrine and other sympathomimetic drugs in patch testing. Methods: We report three cases of allergic contact reactions (dermato-conjunctivitis) after eyedrops. Skin prick tests, epicutaneous testing with the implicated drugs, additives, and a complete patch test battery, TRUE test (Upjohn-Pharmacia, Sweden), were performed in each patient. Results: All skin prick tests were negative. The three patients showed positive patch tests to phenylephrine and one of them also to ephedrine. Tolerance of the other eyedrops without phenylephrine was verified by challenge. Conclusion: Phenylephrine was the responsible agent for the reactions in our patients as confirmed by clinical findings and positive patch tests. Our findings suggest the central structure as the sensitizing part of drug in the second patient. Patch testing is essential for diagnosis of allergic contact reactions of and around the eye.
Rajaei, H., Hezave, A.Z., Lashkarbolooki, M. and Esmaeilzadeh, F., 2013. Representing experimental solubility of phenylephrine hydrochloride in supercritical carbon dioxide and modeling solute solubility using semi-empirical correlations. The Journal of Supercritical Fluids, 75, pp.181-186.
Abstract. Phenylephrine is used as a decongestant sold as an oral medicine, as a nasal spray, or as eye drops. Phenylephrine is now the most common over-the-counter decongestant in the United States. In this regard, measuring the solubility of phenylephrine hydrochloride seems applicable in supercritical carbon dioxide-based processes dealing with this drug. The obtained solubility data obtained by a static method coupled with gravimetric method were in the range of 1.01 × 10−4 to 2.89 × 10−3 based on the mole fraction at the different temperature and pressure ranges of 308.15–338.15 K and 160–400 bar, respectively. In addition, the solubility data were used to obtain the adjusting parameters of semi-empirical correlations namely Kumar and Johnston, Bartle et al., Chrastil and Mendez-Santiago-Teja using a simple data regression.
Temesvári E, Pónyai G, Németh I, Hidvégi B, Sas A, Kárpáti S. Periocular dermatitis: a report of 401 patients. J Eur Acad Dermatol Venereol. 2009 Feb;23(2):124-8. doi: 10.1111/j.1468-3083.2008.02949.x.
Abstract. Background: Periocular contact dermatitis may appear as contact conjunctivitis, contact allergic and/or irritative eyelid and periorbital dermatitis, or a combination of these symptoms. The clinical symptoms may be induced by several environmental and therapeutic contact allergens. Objectives: The aim of the present study was to map the eliciting contact allergens in 401 patients with periocular dermatitis (PD) by patch testing with environmental and ophthalmic contact allergens. Methods: Following the methodics of international requirements, 401 patients were tested with contact allergens of the standard environmental series, 133 of 401 patients with the Brial ophthalmic basic and supplementary series as well. Results: Contact hypersensitivity was detected in 34.4% of the patients. Highest prevalence was seen in cases of PD without other symptoms (51.18%), in patients of PD associated with ophthalmic complaints (OC; 30.4%), and PD associated with atopic dermatitis (AD; 27.9%). In the subgroup of PD associated with seborrhoea (S) and rosacea (R), contact hypersensitivity was confirmed in 17.6%. Most frequent sensitisers were nickel sulphate (in 8.9% of the tested 401 patients), fragrance mix I (4.5%), balsam of Peru (4.0%), paraphenylendiamine (PPD) (3.7%), and thiomersal (3.5%). By testing ophthalmic allergens, contact hypersensitivity was observed in nine patients (6.7% of the tested 133 patients). The most common confirmed ophthalmic allergens were cocamidopropyl betaine, idoxuridine, phenylephrine hydrochloride, Na chromoglycinate, and papaine. Limitations: Patients with symptoms of PD were tested from 1996 to 2006. Conclusions: The occurence of contact hypersensitivity in PD patients was in present study 34.4%. A relatively high occurrence was seen in cases of PD without other symptoms, in PD + OC and in PD + AD patients. The predominance of environmental contact allergens was remarkable: most frequent sensitizers were nickel sulphate, fragrance mix I, balsam of Peru, thiomersal, and PPD. The prevalence of contact hypersensitivity to ophthalmic allergens did not exceed l.5%.
Akita H, Akamatsu H, Matsunaga K. Allergic contact dermatitis due to phenylephrine hydrochloride, with an unusual patch test reaction. Contact Dermatitis. 2003 Nov;49(5):232-5. doi: 10.1111/j.0105-1873.2003.0250.x.
Abstract. 2-day (2-D) closed patch tests are often used in daily clinical practice and useful for evaluating the cause of allergic contact dermatitis. However, even when 2-D closed patch tests at appropriate concentrations are performed for suspected allergic contact dermatitis based on clinical findings, positive reactions are not always obtained. Therefore, although the use of the allergen again induces similar symptoms, a definite diagnosis cannot be made in some cases. We report a case of allergic contact dermatitis due to phenylephrine hydrochloride in eyedrops, with an unusual patch test reaction. Although the results of the routine 2-D closed patch test were negative, a definite diagnosis could be made by closed scratch-patch test. In addition, long-lasting allergic patch test reactions were observed at the positive scratch-patch test site for about 3 months. We speculated that these unusual results on patch testing in our case were associated with the degree of percutaneous absorption of causative agents. Therefore, even when 2-D closed patch tests are negative, scratch-patch tests may be indicated for patients in whom clinical symptoms continue strongly to suggest contact dermatitis.
Herbst RA, Uter W, Pirker C, Geier J, Frosch PJ. Allergic and non-allergic periorbital dermatitis: patch test results of the Information Network of the Departments of Dermatology during a 5-year period. Contact Dermatitis. 2004 Jul;51(1):13-9. doi: 10.1111/j.0105-1873.2004.00334.x.
Abstract. Periorbital dermatitis is common and can be due to the external use of ophthalmic drugs. We evaluated patch test results of the Information Network of the Departments of Dermatology. During a 5-year period (1995-99), of a total 49,256 patch-tested patients, 1053 (2.1%) were eventually diagnosed as allergic periorbital contact dermatitis (APD) and 588 (1.2%) as non-allergic periorbital dermatitis (NAPD). Patient characteristics between APD, NAPD and other cases (OCs) differed with respect to sex (19.7% male in both periorbital groups versus 36.3% in OCs), atopic dermatitis (10.4% in APD versus 60.2% in NAPD versus 16.9% in OCs) and age, APD being substantially more often (68.2%) aged 40 and above than NAPD (52.6%). Several of the top allergens in OCs [such as fragrance mix, Myroxylon pereirae resin (balsam of Peru), lanolin alcohol and potassium dichromate] caused significantly fewer positive test reactions in both periorbital groups. In contrast, thimerosal, phenylmercuric acetate, sodium disulfite, gentamicin sulfate, phenylephrine hydrochloride and benzalkonium chloride tested positively significantly more often in APD but not in NAPD, verifying them as true ophthalmic allergens. Finally, in 42 cases (4%) of APD patients, additional allergens were identified by testing of the patients' own substances (mostly beta-blockers, oxybuprocaine and dexpanthenol), supporting the necessity of testing with ophthalmic drugs as is where individual substances are not readily available.
Sánchez-González JM, Flikier D, Nebro-Cobos S, Zamorano-Martín F, Rachwani-Anil R, García-Lorente M, Borroni D, Peraza-Nieves J, Rocha-de-Lossada C. The Combined Effect of Tropicamide and Phenylephrine on Corneal Astigmatism Axis. Curr Eye Res. 2022 Feb;47(2):179-186. doi: 10.1080/02713683.2021.1971720.
Abstract. Purpose: To analyze astigmatism axis changes after tropicamide and phenylephrine combined instillation. Method: One hundred and thirty-one eyes from 66 patients enrolled this cross-sectional study. An extensive ocular examination was carried out prior to tropicamide and phenylephrine instillation. Power and axis value from flat, steep, and mean keratometry were calculated using an Auto Kerato-Refractometer (AKR). Later, topography and tomography maps were evaluated with Pentacam HR® (Oculus, Wetzlar, Germany). Subsequently, a single drop of tropicamide 1% and phenylephrine hydrochloride 10% were instilled twice, with a five-minute gap between each instillation. After 30 minutes, the AKR and Pentacam HR® tests were repeated. Results: Incyclotorsion was found in 59 eyes (45.1%) and mean absolute incyclotorsion change was 3.91 ± 3.62 degrees (0.10 to 14.20). Excyclotorsion was found in 72 eyes (54.9%) and mean excyclotorsion change was 4.99 ± 5.94 degrees (0.20 to 36.20). We observed that 74.6% and 68.1% of eyes experienced incyclotorsion and excyclotorsion within 0 to 5 degrees, respectively. Fewer patients experienced incyclotorsion and excyclotorsion changes within 5 to 10 degrees, precisely 11.8% and 19.4%, respectively. Eyes that experienced over 10 degrees of incyclotorsion and excyclotorsion were 13.6% and 12.5%, respectively. Conclusion: Astigmatism axis could change after combined tropicamide and phenylephrine instillation. Reference axis marking in astigmatism correction surgery should be performed under the same circumstances as the astigmatism axis has been measured.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Phenylephrine hydrochloride Review Consensus 10 by Frank123 (12058 pt) | 2023-May-14 11:37 |
| Read the full Tiiip | (Send your comment) |
Phenylephrine hydrochloride is a synthetic sympathomimetic chemical compound structurally similar to epinephrine and is produced using an asymmetric Sharpless dihydroxylation of 3-hydroxybenzaldehyde
Phenylephrine is an amine, an organic derivative of ammonia, belonging to the phenylethanamine class, a selective alpha-1 adrenoreceptor agonist with strong vasoconstrictive properties.
What it is used for and where
Medical
Phenylephrine hydrochloride is a mydriatic agent that acts with selective stimulation on the alpha-1 receptor and is used to dilate the iris through stimulation of the iris dilator muscle. It is also used as a spray or oral medication for nasal decongestion.
During spinal and inhalation anaesthesia, it is used to treat vascular insufficiency by maintaining an adequate blood pressure level (1), to prolong spinal anaesthesia and as a vasoconstrictor in regional analgesia.
Cosmetics
It is a restricted ingredient as II/21 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009
Safety
This study reports a case of phenylephrine hydrochloride-induced periorbital contact dermatitis with keratoconjunctivitis (2) and other studies confirm cases of allergic contact reactions from phenylephrine hydrochloride (3), which is considered to be one of the most common confirmed ophthalmic allergens (4) and an ophthalmic allergen (5). It would therefore be advisable to perform a patch test before putting phenylephrine hydrochloride drops in the eyes to ensure that no allergic problems arise. It should be noted, however, that some conjunctivitis may turn out not to be allergic, but caused by bacteria, usually if yellow serum emission is present and the eyelids stick together. Always, of course, ask the treating physician.
Su questo ingrediente sono stati selezionati gli studi più rilevanti con una sintesi dei contenuti:
"Phenylephrine hydrochloride studies"
 |  |
 |  |
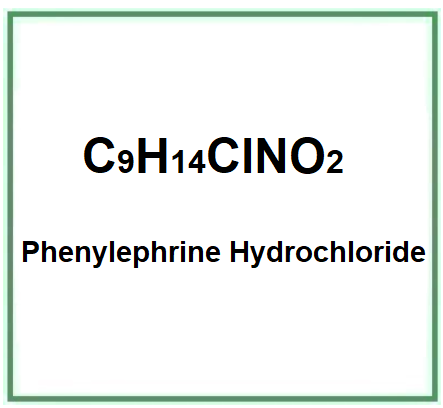
- Molecular Formula C9H14ClNO2
- Molecular Weight 203.66
- CAS 61-76-7
- UNII 04JA59TNSJ
- EC Number 200-517-3
- IUPAC Phenylephrine hydrochloride
References_____________________________________________________________________
(1) Rajaei, H., Hezave, A.Z., Lashkarbolooki, M. and Esmaeilzadeh, F., 2013. Representing experimental solubility of phenylephrine hydrochloride in supercritical carbon dioxide and modeling solute solubility using semi-empirical correlations. The Journal of Supercritical Fluids, 75, pp.181-186.
(2) Kato M, Nitta K, Kano Y, Yamada M, Ishii N, Hashimoto T, Ohyama M. Case of phenylephrine hydrochloride-induced periorbital contact dermatitis with fulminant keratoconjunctivitis causing pseudomembrane formation. J Dermatol. 2018 Feb;45(2):e27-e28. doi: 10.1111/1346-8138.14077.
(3) Moreno-Ancillo A, Munoz-Robles ML, Cabañas R, Barranco P, Lopez-Serrano MC. Allergic contact reactions due to phenylephrine hydrochloride in eyedrops. Ann Allergy Asthma Immunol. 1997 Jun;78(6):569-72. doi: 10.1016/s1081-1206(10)63217-6.
(4) Temesvári E, Pónyai G, Németh I, Hidvégi B, Sas A, Kárpáti S. Periocular dermatitis: a report of 401 patients. J Eur Acad Dermatol Venereol. 2009 Feb;23(2):124-8. doi: 10.1111/j.1468-3083.2008.02949.x.
(5) Herbst RA, Uter W, Pirker C, Geier J, Frosch PJ. Allergic and non-allergic periorbital dermatitis: patch test results of the Information Network of the Departments of Dermatology during a 5-year period. Contact Dermatitis. 2004 Jul;51(1):13-9. doi: 10.1111/j.0105-1873.2004.00334.x.
Abstract. Periorbital dermatitis is common and can be due to the external use of ophthalmic drugs. We evaluated patch test results of the Information Network of the Departments of Dermatology. During a 5-year period (1995-99), of a total 49,256 patch-tested patients, 1053 (2.1%) were eventually diagnosed as allergic periorbital contact dermatitis (APD) and 588 (1.2%) as non-allergic periorbital dermatitis (NAPD). Patient characteristics between APD, NAPD and other cases (OCs) differed with respect to sex (19.7% male in both periorbital groups versus 36.3% in OCs), atopic dermatitis (10.4% in APD versus 60.2% in NAPD versus 16.9% in OCs) and age, APD being substantially more often (68.2%) aged 40 and above than NAPD (52.6%). Several of the top allergens in OCs [such as fragrance mix, Myroxylon pereirae resin (balsam of Peru), lanolin alcohol and potassium dichromate] caused significantly fewer positive test reactions in both periorbital groups. In contrast, thimerosal, phenylmercuric acetate, sodium disulfite, gentamicin sulfate, phenylephrine hydrochloride and benzalkonium chloride tested positively significantly more often in APD but not in NAPD, verifying them as true ophthalmic allergens. Finally, in 42 cases (4%) of APD patients, additional allergens were identified by testing of the patients' own substances (mostly beta-blockers, oxybuprocaine and dexpanthenol), supporting the necessity of testing with ophthalmic drugs as is where individual substances are not readily available.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2023-05-02 19:10:02 | Chemical Risk: |