![]() Kathon
Kathon
Rating : 4
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
10 pts from Frank123
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Kathon studies" about Kathon Review Consensus 10 by Frank123 (12058 pt) | 2023-May-07 11:56 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Pirmez, R., Fernandes, A.L.C. and Melo, M.G.M., 2015. Photoaggravated contact dermatitis to Kathon CG (methylchloroisothiazolinone/methylisothiazolinone): a novel pattern of involvement in a growing epidemic?. British Journal of Dermatology, 173(5), pp.1343-1344.
Abstract. The combination of methylchloroisothiazolinone (MCI) and methylisothiazolinone (MI), commonly available as Kathon™ CG (Dow Chemical Company, Midland, MI, U.S.A.), is widely used as a preservative in cosmetic, household and industrial products.1 2 It was first introduced to Europe in the mid‐1970s, and in 1980 to the U.S.A. Since then an increasing trend of sensitization to MCI/MI and to MI alone (which is also used in cosmetics) has been reported to the point of it being considered an epidemic.2 3 As patients are exposed daily to different products containing MCI/MI, any part of the body can be affected and clinical manifestations are quite variable.
While investigating patients presenting lesions in a photodistributed pattern, a trend towards positive patch testing to MCI/MI was noted in our clinic. Retrospective review of medical records and clinical images from the last 4 years revealed 27 patients with allergic contact dermatitis (ACD) to MCI/MI presenting such a pattern. Photoaggravated ACD to MCI/MI was confirmed in a further three patients through photopatch testing.
Towle KM, Drechsel DA, Warshaw EM, Fung ES, Novick RM, Paustenbach DJ, Monnot AD. Risk Assessment of the Skin Sensitization Induction Potential of Kathon CG in Rinse-off and Leave-on Personal Care and Cosmetic Products. Dermatitis. 2018 May/Jun;29(3):132-138. doi: 10.1097/DER.0000000000000359.
Abstract. Background: Kathon CG is a commonly used cosmetic-grade preservative that contains active ingredients methylchloroisothiazolinone (MCI) and methylisothiazolinone (MI). Objective: The aim of the study was to perform a skin sensitization induction risk assessment of daily exposure to Kathon CG after use of various personal care and cosmetic products. Methods: We calculated an estimated daily consumer exposure level for rinse-off and leave-on products using the amount of product applied per application, number of applications per day, a retention factor, the MCI/MI concentration, and body surface area values. We assumed that the products contained the maximum recommended safe concentration of MCI/MI: 15 ppm in rinse-off products and 7.5 ppm in leave-on products. We compared estimated consumer exposure levels with the no expected sensitization induction level for MCI/MI and applied sensitization assessment factors to calculate product-specific margins of safety (MOSs). Conclusions: The MOSs for rinse-off products ranged from 5 to 63, whereas the MOSs for leave-on products ranged from 0.03 to 1.49. Overall, our results provide evidence that some leave-on products containing the maximum recommended safe concentration of Kathon CG may increase the risk of sensitization induction due to exposure to MCI/MI. In contrast, rinse-off products were not associated with a potential increased risk of skin sensitization induction.
Connor, T.H., Tee, P.G., Afshar, M. and Connor, K.M., 1996. Mutagenicity of cosmetic products containing Kathon®. Environmental and molecular mutagenesis, 28(2), pp.127-132.
Abstract. A variety of shampoos, conditioners, skin-care lotions, and other cosmetic products contain the biocide Kathon® CG, which is a mixture of two heterocyclic isothiazolinones: methylisothiazolinone and methylchloroisothiazolinone. This mixture and the related biocide, Kathon® 886, have been shown to be potent sensitizers and bacterial mutagens. Five cosmetic products that list the components of Kathon® on their labels and two that do not were screened for mutagenicity with Salmonella typhimurium TA100 without S-9. Five of these products and Kathon® 886 were further evaluated in TA100 without and with S-9. Kathon® 886, a cosmetic product that contained Kathon®, and thin layer chromatography-separated components of Kathon® 886 were identified by GC/MS analysis. Three of the five products that listed Kathon® were direct acting mutagens with TA100. The remaining two products were considerably more toxic than the other products and could not be evaluated for mutagenicity. The addition of S-9 reduced toxicity but did not eliminate mutagenicity. The mutagenic evaluation of Kathon® 886 resulted in a dose response similar to that seen with some cosmetic products but at a 1,000-fold lower concentration, and activity was also reduced by the addition of S-9 mix. S-9 reduced activity both with and without cofactors present. Thin layer chromatography separation of the components and subsequent identification by GC/MS indicated that methylisothiazolinone was nonmutagenic while methylchloroisothiazolinone was mutagenic. Additionally, a dichlorinated compound was identified which was also mutagenic. In light of these findings and the reported skin sensitization by Kathon® CG in various cosmetics, we recommend that additional testing be done to assure the safety of products containing Kathon® CG. © 1996 Wiley-Liss, Inc.
Nicoletti, G., Boghossian, V., Gurevitch, F., Borland, R. and Morgenroth, P., 1993. The antimicrobial activity in vitro of chlorhexidine, a mixture of isothiazolinones (‘Kathon’CG) and cetyl trimethyl ammonium bromide (CTAB). Journal of hospital infection, 23(2), pp.87-111.
Abstract. Chlorhexidine, two 4% chlorhexidine antiseptic hand-washes (‘Bioprep’ and ‘Hibiclens’), cetyl trimethyl ammonium bromide (CTAB) and isothiazolinones (‘Kathon’) were tested against Staphylococcus aureus, Micrococcus luteus, Escherichia coli, Serratia marcescens, Pseudomonas aeruginosa, Proteus vulgaris and Candida albicans. The activities measured were the minimum inhibitory concentration (MIC), minimum microbicidal concentration (MMC), rate of kill in water and broth, effect of organic soil, the development of microbial resistance on continuous exposure and agent bioavailability in media and formulation. ‘Kathon’ was the most active microbistatic agent showing maximal activity at low concentration, least inactivation by organic soil and media components and the lowest level of development of bacterial resistance. It was synergistic with chlorhexidine against S. marcescens and P. aeruginosa. Media, formulation components and organic soil affected the performance of chlorhexidine and CTAB. Chlorhexidine was more broadly active than CTAB but showed a greater reduction in activity in the presence of soil and engendered a greater level of bacterial resistance. It was more rapidly bactericidal to P. aeruginosa and S. marcescens than to S. aureus. Stable resistance to chlorhexidine and CTAB was developed by P. aeruginosa and S. marcescens, the latter showing the higher level of resistance. Chlorhexidine-resistant strains were also resistant to CTAB. The antiseptic formulations were more rapidly bactericidal than chlorhexidine alone but were otherwise of comparable activity. Mixtures of disinfectants, in particular a combination of chlorhexidine and a preservative level of ‘Kathon’, were more active than single disinfectants. The importance of standardization of media and test conditions and the use of chemically defined media for accurate and reproducible in-vitro testing of disinfectant activity is emphasized. Disinfection kinetics, expressed as time-kill curves, log reduction factors or decimal reduction times were shown to be valuable in differentiating microbistatic from microbicidal activity, showing the effects of dilution and soil on activity and indicating possible different mechanisms of action.
de Groot, A.C. and Weyland, J.W., 1988. Kathon CG: a review. Journal of the American Academy of Dermatology, 18(2), pp.350-358.
Abstract. Kathon CG, a cosmetics preservative containing, as active ingredients, 5-chloro-2-methyl-4-isothiazolin-3-one and 2-methyl-4-isothiazolin-3-one, appears to be a frequent cause of contact dermatitis in Europe. In the United States, where Kathon CG was introduced some 5 years later, the use of this preservative system for cosmetics and toiletries is rapidly increasing. Undoubtedly cases of contact sensitization will soon emerge in this country. Most cases of contact allergy are caused by the use of moisturizing creams on (slightly) damaged skin. Sensitization by the use of cosmetic products on previously healthy skin, especially the face, does occur but appears to be less frequent. Rinse-off products do not seem to have a substantial potential for the induction and elicitation of contact allergic reactions to Kathon CG because of dilution of the product and the allergen with water as well as a short contact time with the skin. This review provides a synopsis of current knowledge on the preservative system Kathon CG, with emphasis on the risk of sensitization and diagnostic procedures.
Cronin, E., Hannuksela, M., Lachapelle, J.M., Maibach, H.I., Malten, K.E. and Meneghini, C.L., 1988. Frequency of sensitisation to the preservative Kathon® CG. Contact Dermatitis, 18(5), pp.274-279.
Abstract. The incidences of sensitisation to Kathon® CG are reported for patients routinely tested from 1984 to 1986 in Bari. London. Louvain, Nijmegen, Oulu and San Francisco, For these 3 years, the overall frequency of sensitisation varied for women from 3.3% in Oulu to 0.6% in London and San Francisco and for men from 1.4% in Oulu to 0% in San Francisco. Women were predominantly sensitised, probably by cosmetics, toiletries and domestic cleaners. Occupational sensitisation was much less frequent.
Menné, T. and Hjorth, N., 1988. Kathon® CG reactivity in 1396 consecutively patch tested patients in the Copenhagen area. Contact Dermatitis, 19(4), pp.260-262.
Abstract. In 1396 consecutively patch tested patients 18 (l.3%) reacted to Kathon® CG. Relevance was established in 4 of the 18 patients. The frequency or positive reactions to Kathon® CG in patients eczema patients seems to have beers stable in Denmark during the period 1983 to 1988.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Kathon Review Consensus 10 by Frank123 (12058 pt) | 2023-Aug-02 21:41 |
| Read the full Tiiip | (Send your comment) |
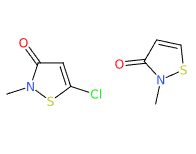
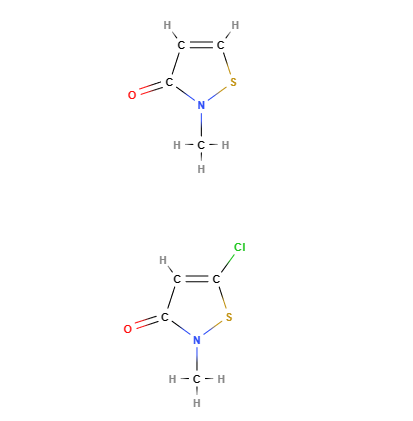
Kathon® is a chemical compound, a mixture of two heterocyclic isothiazolinones: methylchloroisothiazolinone and methylisothiazolinone included in the formulation in approximate proportions of 3:1 and with magnesium chloride and magnesium nitrate as stabilisers. It is a registered trademark of Rohm and Haas Company.
Name breakdown and function of the components
- Kathon - A trade name referring to a mix of two isothiazolinones, specifically methylchloroisothiazolinone (MCI) and methylisothiazolinone (MI). These compounds are primarily used as preservatives.
Isothiazolinones, and specifically the Kathon mix, are employed as biocides in a range of products, including personal care items, due to their effective antimicrobial properties.
Description and function of the raw materials used in production
- Isothiazolinones - Chemical compounds that have the ability to inhibit microbial growth and hence are used as preservatives.
Summary of the industrial production process step by step
- Chemical Synthesis - Isothiazolinones are produced through a series of chemical reactions.
- Formulation - The isothiazolinones are then combined in specific proportions to create the Kathon mix.
- Purification - The resulting mix might be purified to ensure removal of impurities.
- Packaging - Finally, the Kathon mix is packaged for distribution and use in end products.
It appears as a colourless transparent liquid.

What it is used for and where
It is a highly efficient, broad-spectrum, non-oxidising antibacterial, stable at acid pH and in more oxidising environments. It is commercially available under different names: Kathon 886, Kathon CG, Kathon LX and others.
Cosmetics
Preservative. Any product containing organic, inorganic compounds, water, needs to be preserved from microbial contamination. Preservatives act against the development of harmful microorganisms and against oxidation of the product.
Food
Chemical preservative that prevents the degradation of milk samples and maintains the authenticity of analysis results.
Safety
Studies published so far attribute contact allergies, contact dermatitis (1) and, if inhaled, risk of eosinophilia-mediated disease in the lung to Kathon (2)
Commercial applications
Cosmetic products: Used as a preservative in many cosmetic products such as creams, lotions, shampoos, and bath gels.
Cleaning products: It's also utilized in cleansers, soaps, and other cleaning products to stave off microbial growth.
Industrial products: Found in certain paints, adhesives, and other industrial products as a preservative.
Properties
Preservative: Helps prevent the growth of bacteria, fungi, and other microorganisms in products.
Biocidal: Possesses biocidal properties that kill microorganisms.
Compendium of the most significant studies with reference to properties, intake, effects.
 |  |
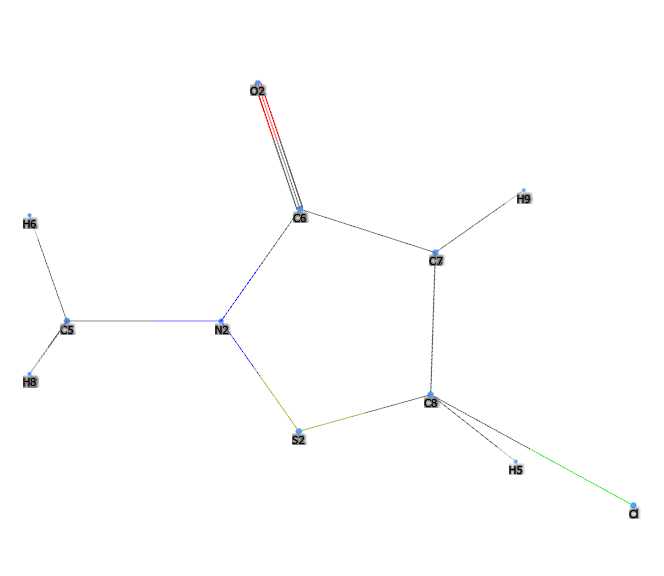 | 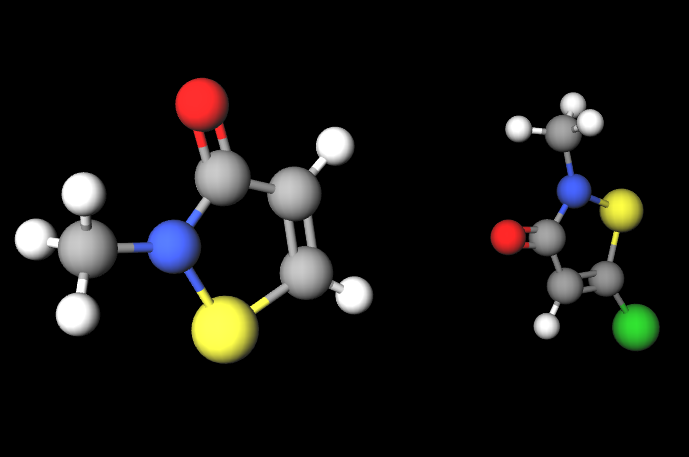 |
- Molecular Formula C8H9ClN2O2S2 C4H4ClNOS
- Molecular Weight 264.8
- CAS 55965-84-9 (Kathon 886), 5-chloro-2- methyl-4-isothiazolin-3-one (methylchloroisothiazolinone) 26172-55-4, 2-methyl-4 isothiazolin-3-one (methylisothiazolinone) 2682-20-4
- UNII 15O9QS218W
- EC Number 911-418-6 611-341-5 932-593-5 247-500-7
References_____________________________________________________________________
(1) Kazandjieva, J., Gergovska, M. and Darlenski, R., 2014. Contact dermatitis in a child from methlychloroisothiazolinone and methylisothiazolinone in moist wipes. Pediatric Dermatology, 31(2), pp.225-227.
Abstract. Contact allergic reactions to methlychloroisothiazolinone/methylisothiazolinone also widely known as Kathon CG have been reported extensively reported. It is one of the most commonly used preservatives in rinse-off products, cosmetics, and others. Herein, a case of a 50-year-old girl is presented with chronic dermatitis in the anogenital area. The patient was patch tested and had positive reaction to Kathon CG. The detailed history taking revealed that the allergen was present in the moist cleaning wipes used instead of dry toilet paper. The presented case serves as a basis for a appraisal of the use of this preservative in wet wipes. In addition, the duration of the patch test protocol in children has also been discussed.
(2) Park, E.J., Han, J.S., Seong, E., Park, E.J., Lee, B.S., Lee, S.J. and Lee, K., 2020. Inhaled Kathon may induce eosinophilia‐mediated disease in the lung. Environmental toxicology, 35(1), pp.27-36.
Abstract. In 2011, a link between humidifier disinfectants and patients with idiopathic pulmonary fibrosis was identified in Korea, and Kathon was suggested as one of the causative agents. In this study, Kathon induced apoptotic cell death along with membrane damage at 24 h post-exposure. Additionally, on day 14 after a single instillation with Kathon, the total number of pulmonary cells and the levels of TNF-α, IL-5, IL-13, MIP-1α, and MCP-1α clearly increased in the lung of mice. The proportion of natural killer cells and eosinophils were significantly elevated in the spleen and the bloodstream, respectively, and the level of immunoglobulin (Ig) A, but not IgG, IgM, and IgE, dose-dependently increased. Therefore, we suggest that inhaled Kathon may induce eosinophilia-mediated disease in the lung by disrupting homeostasis of pulmonary surfactants. Considering that eosinophilia is closely related to cancer and fibrosis, further studies are needed to understand the relationship between them.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2023-05-07 12:10:35 | Chemical Risk: |