Hydroxyacetophenone is an organic chemical intermediate compound, a natural product found in plants. Used in cosmetics and skincare products as an antioxidant, stabilizing, and soothing agent. With properties that enhance formulation stability and provide calming benefits to the skin, Hydroxyacetophenone is particularly valued in products designed for sensitive or stressed skin.
The name describes the structure of the molecule:
- "Hydroxy": This refers to the presence of a hydroxyl group (-OH) in the molecule.
- "Aceto": This is derived from "acetyl", which is a functional group characterized by the structure -COCH3.
- "Phenone": This indicates the presence of a carbonyl group (C=O) attached to a benzene ring, which is a six-carbon ring with alternating double bonds.
So, Hydroxyacetophenone is a molecule that contains a benzene ring with a carbonyl group (forming a phenone), an acetyl group, and a hydroxyl group.
The synthesis of hydroxyacetophenone can be achieved through the Friedel-Crafts acylation of phenol with acetic anhydride in the presence of a Lewis acid catalyst such as aluminum chloride (AlCl3).
The synthesis process takes place in different steps:
- Phenol (C6H5OH) is reacted with acetic anhydride ((CH3CO)2O) in the presence of a Lewis acid catalyst, aluminum chloride (AlCl3).
- The aluminum chloride catalyzes the reaction by making the acetic anhydride more electrophilic, allowing it to react with the phenol.
- The acetyl group from the acetic anhydride attaches to the benzene ring of the phenol, forming hydroxyacetophenone and acetic acid as a byproduct.
Industrially it appears as a white powder that is insoluble in water.

What it is used for and where
Medical
Hydroxyacetophenone (4-Hydroxyacetophenone) inhibits adhesion, invasion and migration of colon cancer cells in vitro and reduces the metastatic burden in an in vivo model of colon cancer metastasis to the liver (1).
Cosmetics
Antioxidant agent. Ingredient that counteracts oxidative stress and prevents cell damage. Free radicals, pathological inflammatory processes, reactive nitrogen species and reactive oxygen species are responsible for the ageing process and many diseases caused by oxidation.
Safety
Hydroxyacetophenone is a compound generally considered safe, but, as with all chemical compounds, it is possible to develop allergic reaction as in the case described in this study (2).
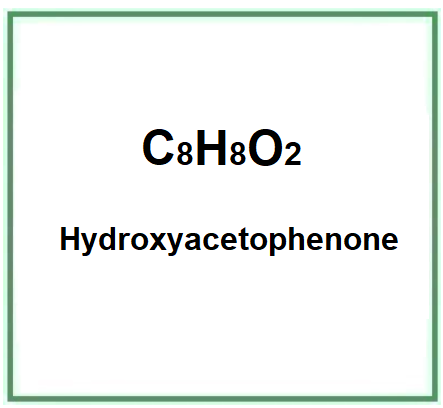
- Molecular Formula C8H8O2
- Molecular Weight 99-93-4
- CAS 99-93-4
- UNII G1L3HT4CMH
- EC Number 202-802-8
Synonyms:
References_____________________________________________________________________
(1) Bryan DS, Stack M, Krysztofiak K, Cichoń U, Thomas DG, Surcel A, Schiffhauer ES, Beckett MA, Khodarev NN, Xue L, Poli EC, Pearson AT, Posner MC, Robinson DN, Rock RS, Weichselbaum RR. 4-Hydroxyacetophenone modulates the actomyosin cytoskeleton to reduce metastasis. Proc Natl Acad Sci U S A. 2020 Sep 8;117(36):22423-22429. doi: 10.1073/pnas.2014639117.
Abstract. Metastases are the cause of the vast majority of cancer deaths. In the metastatic process, cells migrate to the vasculature, intravasate, extravasate, and establish metastatic colonies. This pattern of spread requires the cancer cells to change shape and to navigate tissue barriers. Approaches that block this mechanical program represent new therapeutic avenues. We show that 4-hydroxyacetophenone (4-HAP) inhibits colon cancer cell adhesion, invasion, and migration in vitro and reduces the metastatic burden in an in vivo model of colon cancer metastasis to the liver. Treatment with 4-HAP activates nonmuscle myosin-2C (NM2C) (MYH14) to alter actin organization, inhibiting the mechanical program of metastasis. We identify NM2C as a specific therapeutic target. Pharmacological control of myosin isoforms is a promising approach to address metastatic disease, one that may be readily combined with other therapeutic strategies.
(2) Sanz-Sánchez T, Valverde Garrido R, Maldonado Cid P, Díaz-Díaz RM. Allergic contact dermatitis caused by hydroxyacetophenone in a face cream. Contact Dermatitis. 2018 Feb;78(2):174-175. doi: 10.1111/cod.12900. PMID: 29341188.
![]() Hydroxyacetophenone
Hydroxyacetophenone