![]() Bronopol
Bronopol
Rating : 5
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Antibacterial (1)Cons:
Allergen (1)10 pts from A_Partyns
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about Bronopol Review Consensus 10 by A_Partyns (12948 pt) | 2024-Oct-08 16:21 |
| Read the full Tiiip | (Send your comment) |
Bronopol, commonly known as 2-Bromo-2-Nitropropane-1,3-Diol, is a preservative used in cosmetic, pharmaceutical, and industrial applications. It is a highly effective antimicrobial agent, primarily used to control bacterial, fungal, and yeast growth in various formulations. Its inclusion in personal care products helps to extend shelf life by preventing microbial contamination. However, Bronopol is a restricted ingredient (Annex V/21) under the European Cosmetics Regulation 1223/2009 due to concerns about its potential to release formaldehyde under certain conditions.
Chemical Composition and Structure
Bronopol (C₃H₆BrNO₄) is composed of bromine, nitrogen, and oxygen atoms attached to a propane diol structure. It acts as an antimicrobial by interfering with microbial cell function and disrupting growth. Its effectiveness in water-based formulations makes it popular in cosmetics, skincare products, and pharmaceuticals.
Physical Properties
Bronopol is a white to off-white crystalline powder that is highly soluble in water, which allows for easy incorporation into a wide range of products. It is thermally stable under normal conditions, but under elevated temperatures or specific pH conditions, Bronopol can release formaldehyde.
Production Process
Bronopol is synthesized through the bromination of nitropropanediol, followed by a purification process to yield a high-purity product. It is typically manufactured as a fine powder, which can be easily mixed into cosmetic and industrial formulations.
It consists of:
- Hydrogen (H): 6 atoms, 40.0% mol, 3.024% mass
- Oxygen (O): 4 atoms, 26.7% mol, 32.00% mass
- Carbon (C): 3 atoms, 20.0% mol, 18.02% mass
- Nitrogen (N): 1 atom, 6.67% mol, 7.004% mass
- Bromine (Br): 1 atom, 6.67% mol, 39.95% mass

The name defines the structure of the molecule:
- 2-Bromo indicates the presence of a bromine atom attached to the second carbon atom in the compound's carbon chain. "Bromo" is the term used for bromine in a chemical compound.
- 2-Nitro signifies the presence of a nitro group (NO2) also attached to the second carbon atom of the chain. The nitro group is a functional group consisting of nitrogen and oxygen atoms.
- Propane-1,3-Diol is the base molecule of the compound. "Propane" refers to a three-carbon chain. The suffix "-diol" indicates the presence of two alcohol groups (OH) on the molecule. The numbers "1,3" specify that these alcohol groups are attached to the first and third carbon atoms of the propane chain.
The synthesis process takes place in several stages:
- Preparation of nitromethane and propylene oxide. These are the two main reagents used in the synthesis of Bronopol. Nitromethane is typically produced from propane, while propylene oxide can be produced from propylene.
- Reaction of nitromethane and propylene oxide. The two reagents are fed into a reaction vessel. The reaction is exothermic, that is, it releases heat and generally occurs at high temperatures and under pressure.
- Addition of bromo. After the initial reaction, bromine is added to the reaction mixture. This involves bromination of the product, forming 2-Bromo-2-Nitropropane-1,3-Diol.
- Purification. The reaction mixture is purified to isolate Bronopol and this phase includes treatments such as crystallization, filtration and drying.
- Quality control test. The final product is tested to ensure it meets the required specifications. This may involve testing for purity, moisture content and other physical and chemical properties
It occurs as a fine white powder, easily soluble in water, alcohol, propylene glycol, ethyl acetate, slightly soluble in oil and insoluble in acetone and chloroform. Stable under acidic conditions. When the aqueous solution is alkaline, it decomposes slowly and cannot be used with certain metals such as aluminium. It releases formaldehyde. This study found that the decompodsition process producing formaldehyde mainly depends on temperature and pH. Notably, the pH of the Bronopol-diluted buffer solution was the factor most influencing the release of formaldehyde. The release was markedly higher in alkaline buffer compared with acidic buffer. (Kajimura, K., Tagami, T., Yamamoto, T., & Iwagami, S. (2008). The release of formaldehyde upon decomposition of 2-bromo-2-nitropropan-1, 3-diol (bronopol). Journal of health science, 54(4), 488-492.)

What it is used for and where
Cosmetics
It is a restricted ingredient V/21 as a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. This study warns of: "Actual formaldehyde levels from formaldehyde releasers were analysed using a modified HPLC method: Doi et al. detected > 30 mg/kg (> 30 ppm) in 83 of 89 cosmetic product samples containing different FRs, and > 250 ppm in 44/89 samples (3). As summarised in a review by de Groot et al. “… all releasers (with the exception of 2-bromo-2-nitropropane-1,3-diol, for which adequate data are lacking) can, in the right circumstances of concentration and product composition, release >200 ppm formaldehyde, which may result in allergic contact dermatitis.”(Bernauer, U., Bodin, L., Chaudhry, Q., Coenraads, P.J., Dusinska, M., Ezendam, J., Gaffet, E., Galli, C.L., Granum, B.B., Panteri, E. and Rogiers, V., 2021. SCCS SCIENTIFIC ADVICE ON on the threshold for the warning ‘contains formaldehyde’in Annex V, preamble point 2 for formaldehyde-releasing substances-SCCS/1632/21–Scientific advice.)
Preservative. Any product containing organic, inorganic compounds, water, needs to be preserved from microbial contamination. Preservatives act against the development of harmful microorganisms and against oxidation of the product.
First synthesised in 1897, since 1980 it has been used as an antibacterial and antifungal agent to prevent the growth and reproduction of bacteria in personal care products and as a broad-spectrum antibiofilm agent particularly in leave-on and rinse-off shampoos, creams, lotions, rinses and eye make-up. It is added in the formulation of cosmetics such as shampoos, conditioners and creams in a sterilising concentration of 0.01% - 0.02%.
Medical
Since 1960, it has been a broad-spectrum antibacterial and antibiofilm agent and a promising candidate for the treatment of chronic wounds (1).
Other Uses
- Industrial water, plastic, wood: mould prevention, corrosion prevention, algae control as preservative and steriliser.
- Reference standard in ultra-performance liquid chromatography (UPLC) coupled to the coupled plasma mass spectrometry method.
- Inductively for the determination of bromine-containing preservatives from cosmetic products.
- In the agricultural sector against a wide range of plant pathogenic bacteria and is mainly used as a seed treatment agent as well as for the prevention and control of rice seedling diseases against bacterial blight caused by the cotton leaf spot pathogen.
Safety in Use and Regulatory Restrictions
Bronopol is classified as a restricted ingredient (V/21) in the European Cosmetics Regulation 1223/2009 due to its ability to release formaldehyde, a known sensitizer that can trigger allergic contact dermatitis. While Bronopol is an effective preservative, the potential for formaldehyde release, especially in concentrations exceeding 200 ppm, has raised concerns.
In a 2021 review by Bernauer et al., it was noted that formaldehyde levels in products containing formaldehyde releasers (FRs) can exceed 200 ppm under specific circumstances. Doi et al. found that 44 out of 89 cosmetic samples containing various FRs had formaldehyde levels over 250 ppm, which poses a risk for allergic reactions. While other formaldehyde releasers have been studied extensively, adequate data on Bronopol specifically are lacking, adding to regulatory caution.
Allergic Reactions
Bronopol has been associated with skin sensitization, particularly in individuals with formaldehyde allergies or sensitivities. Given the potential for formaldehyde release, products containing Bronopol must be formulated to ensure that the concentration of released formaldehyde remains within safe limits to avoid triggering allergic contact dermatitis.
Toxicity and Carcinogenicity
Formaldehyde, which can be released by Bronopol, is classified as a carcinogen. Therefore, its release in cosmetic products is strictly regulated. While Bronopol itself is not classified as carcinogenic, the formaldehyde release potential under certain conditions (e.g., heat, pH changes) warrants careful formulation to avoid health risks. The Scientific Committee on Consumer Safety (SCCS) has provided scientific advice (SCCS/1632/21) on the threshold for formaldehyde warning labels on products containing formaldehyde-releasing substances.
Environmental and Safety Considerations
Bronopol is biodegradable; however, its potential to release formaldehyde into the environment requires careful consideration in product formulation and disposal. Due to its chemical structure, proper disposal methods are recommended to prevent contamination of water sources.
Regulatory Status
In the European Union, Bronopol is regulated under the Cosmetics Regulation 1223/2009, listed as a restricted substance in Annex V (V/21). Its use is subject to specific concentration limits to minimize formaldehyde release and reduce the risk of allergic reactions. In products containing formaldehyde-releasing preservatives, warnings may be required if the concentration exceeds a specific threshold.
Most significant studies
The aim of this study was to analyse the bacterial community in the production line of a calcium carbonate manufacturing company and to investigate possible causes of bacterial presence. A formulation containing 7.5-15% (v v -1 ) bronopol 1.0-2.5% (v v v -1 ) (chloroisothiazolinone (CIT) + methylisothiazolinone (MIT)) proved to be most effective. Of the possible causes of bacterial presence, sporogenesis and absorption of the biocide to the carbonate particles are the least likely compared to bacterial absorption of the particles and the acquisition of biocide resistance (2).
In terms of allergy, however, according to data from the 2015 - 2016 Pediatric Contact Dermatitis Registry, the top 10 allergens for children (0-5 years) include nickel (42%), peru balsam (19%) (15%), neomycin (17%), formaldehyde (15%), cocamidopropyl betaine (15%), cobalt dichloride (14%), MCI / MI (12%), propylene glycol (9%), bacitracin, bronopol and wool alcohol (8%) (3).
In recent years, Bronopol (2-bromo-2-nitropropane-1,3-diol), a broad-spectrum biocide, has seen use as an effective and economically acceptable alternative in the treatment and control of Saprolegnia sp. a fungus of the family Saprolegniaceae (4), however, concerns are growing regarding its use within industry due to its acute toxicity to several fish species and its potentially harmful effects on human health (5).
Typical commercial product characteristics Bronopol
| Appearance | White crystalline powder |
| Assay | 99.0% – 101.0% |
| Boiling Point | 358.0±42.0 °C at 760 mmHg |
| Melting Point | 130-133°C |
| Flash Point | 170.3±27.9°C |
| Density | 2.0±0.1 g/cm3 |
| pH | 5.0-7.0 |
| Impurity | <0.3% |
| Water | ≤0.5% |
| Sulphated ash | ≤0.1% |
| Water Solubility | 25 g/100 mL (22ºC) |
| PSA | 86.28000 |
| LogP | 1.72 |
| Refraction Index | 1.575 |
| Vapor Pressure | 0.0±1.8 mmHg at 25°C |
| Safety |  |
 |  |
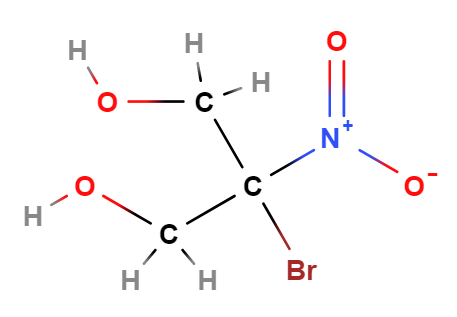 | 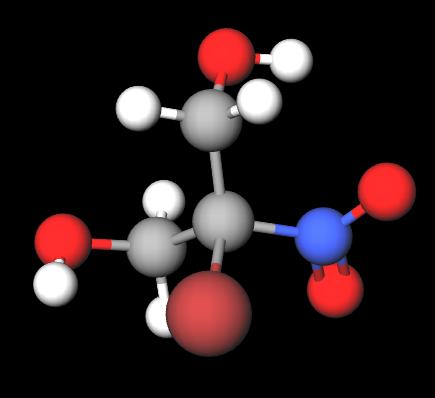 |
- Molecular Formula C3H6O4BrN C3H6BrNO4
- Linear Formula HOCH2CBr(NO2)CH2OH
- Molecular Weight: 199.988 g/mol
- UNII: 6PU1E16C9W
- CAS 52-51-7 133248-96-1 179733-60-9 1135443-73-0
- EC Number: 200-143-0
- DSSTox Substance ID
- IUPAC 2-bromo-2-nitropropane-1,3-diol
- InChI=1S/C3H6BrNO4/c4-3(1-6,2-7)5(8)9/h6-7H,1-2H2
- InChl Key LVDKZNITIUWNER-UHFFFAOYSA-N
- SMILES C(C(CO)([N+](=O)[O-])Br)O
- MDL number MFCD00007390
- PubChem Substance ID 329753983
- ChEBI 31306
- ICSC 0415
- Beilstein 1705868
- NSC 141021
- RTECS TY3385000
- UN 3241
- RXCUI 1114345
Synonyms:
- Bronopol
- 2-Bromo-2-nitro-1,3-propanediol
- 2-Bromo-2-nitropropane-1,3-diol
- 2-Nitro-2-bromo-1,3-propanediol
- 2-bromo-2-nitro-propane-1,3-diol
- 2-bromanyl-2-nitro-propane-1,3-diol
- 1,3-Propanediol,2-bromo-2-nitro-
- .beta.-Bromo-.beta.-nitrotrimethyleneglycol
- Bronosol
- Bronocot
- Bronidiol
- Bronopolu
- beta-Bromo-beta-nitrotrimethyleneglycol
- Bronotak
- Onyxide 500
- Lexgard bronopol
- 1,3-Propanediol, 2-bromo-2-nitro-
- Bioban
- Myacide BT
- InChI=1/C3H6BrNO4/c4-3(1-6,2-7)5(8)9/h6-7H,1-2H2
References_________________________________________________________________
(1) Lee VE, O'Neill AJ. Potential for repurposing the personal care product preservatives bronopol and bronidox as broad-spectrum antibiofilm agents for topical application. J Antimicrob Chemother. 2019 Apr 1;74(4):907-911. doi: 10.1093/jac/dky520.
(2) Odić D, Prah J, Avguštin G. Identification of bacterial contaminants from calcium carbonate filler production lines and an evaluation of biocide based decontamination procedures. Biofouling. 2017 Apr;33(4):327-335. doi: 10.1080/08927014.2017.1310848.
(3) Goldenberg A, Mousdicas N, Silverberg N, Powell D, Pelletier JL, Silverberg JI, Zippin J, Fonacier L, Tosti A, Lawley L, Wu Chang M, Scheman A, Kleiner G, Williams J, Watsky K, Dunnick CA, Frederickson R, Matiz C, Chaney K, Estes TS, Botto N, Draper M, Kircik L, Lugo-Somolinos A, Machler B, Jacob SE. Pediatric Contact Dermatitis Registry Inaugural Case Data. Dermatitis. 2016 Sep-Oct;27(5):293-302. doi: 10.1097/DER.0000000000000214.
(4) Pottinger TG, Day JG. A Saprolegnia parasitica challenge system for rainbow trout: assessment of Pyceze as an anti-fungal agent for both fish and ova. Dis Aquat Organ. 1999 May 12;36(2):129-41. doi: 10.3354/dao036129.
(5) Piamsomboon P., Lukkana M., Wongtavatchai J. Safety and Toxicity Evaluation of Bronopol in Striped Catfish (Pangasianodon hypophthalmus) Thai. J. Vet. Med. 2013;43:477–481
Flores S, Montenegro I, Villena J, Cuellar M, Werner E, Godoy P, Madrid A. Synthesis and Evaluation of Novel Oxyalkylated Derivatives of 2',4'-Dihydroxychalcone as Anti-Oomycete Agents against Bronopol Resistant Strains of Saprolegnia sp. Int J Mol Sci. 2016 Aug 22;17(8):1366. doi: 10.3390/ijms17081366.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (1)
Component type: Natural Main substances: Last update: 2023-06-30 11:01:18 | Chemical Risk: |