E553b or Talc is a chemical compound that is produced directly from talc, steatite, sepiolite, a pressed hydrated magnesium silicate, treated with hydrochloric acid, washed and dried. In its composition are available asbestos, amphiboles, chrysotile, aluminum.
The name defines the structure of the molecule:
- Talc. The name is derived from the Arabic word 'talq', meaning 'pure'. It is a reference to its white colour and the softness of the mineral.
The synthesis process takes place in different stages:
- Mother rock formation. Talc is formed from parent rocks rich in magnesium and silica. The mother rock can be dolomite or serpentine.
- Metamorphism. Over millions of years, these rocks undergo metamorphism, a process in which the mineral composition and structure of the rock are changed by heat and pressure. This process can lead to the formation of talc.
- Extraction and processing Once formed, talc is extracted from open-pit mines and then ground into a fine powder for use.
It comes in the form of a fine, odorless, tasteless, white non-sandy powder with a greasy feel to the touch.

What is it for?
Used in a variety of industries: paints, cosmetics, linoleum, textiles, rubber, paper, plastics, talc is a good modifier for elastomers, improves stiffness and barrier properties. Because of its high specific surface area characteristics, talc is used to dust sticky products, prevents agglomeration and improves handling. Talc has acid and fire resistance, insulation, high melting point, strong absorption strength and is chemically inert because of its crystallization due to its layered structure.
Pharmaceutical
Filling agent, coating of tablets Talc is a gliding excipient, in practice it increases the smoothness of the material in the tablet by reducing the friction between the particles. Typically the concentration is: 0.3-10 %w/w.
Cosmetics
Talc is a restricted ingredient as III/59 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Ingredient at risk: Talc: Hydrated magnesium silicate
- Abrasive agent. It contains abrasive particles to remove stains or biofilm that accumulate on the stratum corneum or teeth. Baking soda, kieselguhr, silica and many others have abrasive properties. Peeling or exfoliating products used in dermatology or cosmetic applications contain abrasive agents in the form of synthetic microspheres, however these microspheres or abrasive particles are not biodegradable and create pollution in aquatic ecosystems.
- Absorbent. Absorbs substances dispersed or dissolved in aqueous solutions, water/oil, oil/water.
- Anticaking agent. This compound facilitates free flow and prevents aggregation or clumping of substances in a formulation by reducing the tendency of certain particles to stick together.
- Bulking agent. It regulates the water content, dilutes other solids, can increase the volume of a product for better flow, acts as a buffer against organic acids, helps to keep the pH of the mixture within a certain level.
- Opacifying agent. It is useful into formulations that may be translucent or transparent to make them opaque and less permeable to light.
- Skin protectant. It creates a protective barrier on the skin to defend it from harmful substances, irritants, allergens, pathogens that can cause various inflammatory conditions. These products can also improve the natural skin barrier and in most cases more than one is needed to achieve an effective result.
- Slip modifier. It increases the spreadability of a product by helping other substances flow more smoothly and easily, without chemical reaction..
Food
Labeled with the number E553b, anti-caking agent, in the European food additives list.
Other uses
Rubber: filling agent (the dosage is 5% of the amount of poly (vinyl acetate)
And also used in printing inks, ceramics, cables, waterproof materials and others
Safety
In recent years, many manufacturers have removed asbestos (a known carcinogenic mineral) from the production of talcum powder because of the dangers that asbestos can create to human health, but some precautions still need to be observed: talcum powder must not come into contact with the horny layer when the latter is damaged.
Among the impurities present in talcum powder processing, aluminium is of some concern. Aluminium can interfere with different biological processes (cellular oxidative stress, calcium metabolism, etc.), so it can induce toxic effects in different organs and systems, and the nervous system is the main target of its toxicity.
Studies
This study finds that exposure to talc in female genitals produces an increase in inflammation resulting in an increased risk of ovarian cancer (1).
Because of the contained asbestos, talc inhalation can cause pulmonary fibrosis in the form of granulomatous nodules called talcosis. Talc exposure has also been suggested as a causative factor in the development of ovarian carcinomas, gynecological cancers and mesothelioma (2).
This study assessed the risk of contracting asbestos-related disease from powdered cosmetic talcum users. The hypothetical treatment of this fiber as if it were an asbestos involves a risk of 9.6 × 10-7 (less than one in a million) (3).
Some cases of talc poisoning (4).
The most relevant studies on this ingredient have been selected with a summary of their contents:
Talc studies
Typical commercial product characteristics
| Appearance | White fine powder |
| Silicon Dioxide, W / % | ≥58.0 |
| Density | 2.7-2.8 g/cm3 |
| pH | 8.0-9.5 |
| Melting Point | 800ºC |
| LOI (at 1050℃) | 13--17% |
| Magnesium Oxide, W / % | ≥30.0 |
| Whiteness | ≥85.0 |
| Acid soluble substance (S04) / % | ≤1.5 |
| Loss on ignition/ % | ≤6.0 |
| Loss on dying / % | ≤0.5 |
| Asbestos | Free |
| Arsenic mg/kg | ≤3 |
| Pb mg/kg | ≤5 |
| Water soluble salt/ % | ≤0.1 |
| Heavy metals(Pb) mg/kg | ≤10 |
| Fineness(45μm), w / % | ≥98.0 |
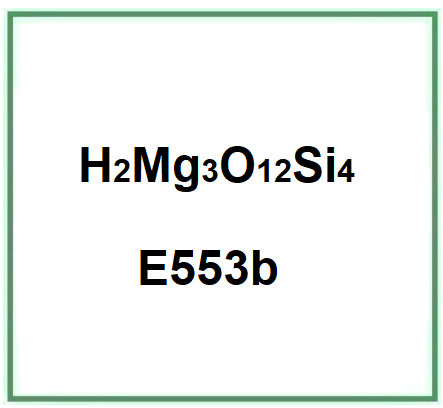
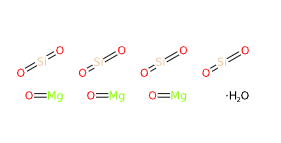
- Molecular Formula : Mg3(OH)2Si4O10 H2Mg3O12Si4 Mg3(Si4O10)(OH)2
- Linear Formula : 3MgO·4SiO2·H2O
- Molecular Weight : 379.259 g/mol
- CAS : 14807-96-6
- UNII 7SEV7J4R1U
- EC Number: 238-877-9
- DSSTox Substance ID: DTXSID5032109
- MDL number MFCD00792903
- PubChem Substance ID
- InChI=1S/3Mg.4O2Si.H2O.3O/c;;;4*1-3-2;;;;/h;;;;;;;1H2;;;
- InChl Key FPAFDBFIGPHWGO-UHFFFAOYSA-N
- SMILES O.O=[Mg].O=[Mg].O=[Mg].O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O
- IUPAC dioxosilane;oxomagnesium;hydrate
- ChEBI
Synonyms :
- Talc CP
- tris(@magnesium oxide) tetrakis(silica) hydrate
- tris(@magnesium oxide) tetrakis(silica) hydrate
- 3MgO.4O2Si.H2O
- E553b
References____________________________________________________________________
(1) Fletcher NM, Harper AK, Memaj I, Fan R, Morris RT, Saed GM. Molecular Basis Supporting the Association of Talcum Powder Use With Increased Risk of Ovarian Cancer. Reprod Sci. 2019 Dec;26(12):1603-1612. doi: 10.1177/1933719119831773.
(2) Gordon RE, Fitzgerald S, Millette J. Asbestos in commercial cosmetic talcum powder as a cause of mesothelioma in women. Int J Occup Environ Health. 2014 Oct;20(4):318-32. doi: 10.1179/2049396714Y.0000000081. Epub 2014 Sep 3. Erratum in: Int J Occup Environ Health. 2015;21(4):347-8.
(3) Anderson EL, Sheehan PJ, Kalmes RM, Griffin JR. Assessment of Health Risk from Historical Use of Cosmetic Talcum Powder. Risk Anal. 2017 May;37(5):918-929. doi: 10.1111/risa.12664.
(4) Pennycuff JF, Davenport A, Ellis J, Patberg E, Cwiak C. Talcum Powder Toxicosis in Pregnancy. AJP Rep. 2018 Oct;8(4):e384-e386. doi: 10.1055/s-0038-1676382.
Palla S, Rangdhol V, Shekar V, Jahan AJ. Oral lichenoid reactions to talcum powder: A usual report with unusual history. Indian J Dermatol Venereol Leprol. 2018 May-Jun;84(3):347-349. doi: 10.4103/ijdvl.IJDVL_159_17.
![]() E553b
E553b