![]() Polyoxyethylene sorbitan monooleate
Polyoxyethylene sorbitan monooleate
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
10 pts from Whiz35
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about Polyoxyethylene sorbitan monooleate Review Consensus 10 by Whiz35 (11840 pt) | 2023-Jul-08 18:22 |
| Read the full Tiiip | (Send your comment) |
Polyoxyethylene sorbitan monooleate, also known as Polysorbate 80, is a non-ionic surfactant and emulsifier often used in food and cosmetics. The name "polyoxyethylene sorbitan monooleate" describes the structure of the molecule:
- "Polyoxyethylene" refers to the presence of multiple (poly) units of oxyethylene in the molecule. Oxyethylene is a chemical compound containing two hydrogen atoms and two oxygen atoms. It is often used in the creation of surfactants, compounds that reduce the surface tension between two liquids or between a liquid and a solid.
- "Sorbitan" is derived from sorbitol, a sugar alcohol that is often used as a sweetener and humectant (a substance that promotes moisture retention). In the context of polyoxyethylene sorbitan monooleate, sorbitan refers to dehydrated sorbitol which is used as the base molecule in the compound.
- "Monooleate" indicates that there is an oleate (mono) group in the molecule. An oleate is a salt or ester of oleic acid, a fatty acid that is naturally found in various animal and vegetable fats and oils.
Thus, polyoxyethylene sorbitan monooleate is a compound that consists of a dehydrated sorbitol (sorbitan) base with multiple oxyethylene units and a single oleate group. It is used as an emulsifier in various products, including food and cosmetics, to help mix oil and water components.
Polyoxyethylene sorbitan monooleate is a compound that consists of a dehydrated sorbitol (sorbitan) base with multiple oxyethylene units and a single oleate group. It's used as an emulsifier in a variety of products, including food and cosmetics, to help mix oil and water components.
The synthesis process takes place in several stages.
- Step 1: Preparation of raw materials: sorbitol, ethylene oxide and oleic acid.
- Step 2: Ethoxylation. Sorbitol reacts with ethylene oxide in a process that involves the addition of ethylene oxide to the sorbitol molecule, creating a series of repeated ethylene oxide units.
- Step 3: Esterification. Sorbitol ethoxylate is reacted with oleic acid and involves the formation of an ester bond between sorbitol ethoxylate and oleic acid, creating the final molecule Polysorbate 80.
What it is used for and where
Cosmetics
It is used as a surfactant, stabilizer and emulsifier in the composition of cosmetics and has these INCI functions:
Denaturant. The ionic or polar molecules of this ingredient included in formulations that interact with protein groups, modulate the properties of the solution to suit specific needs.
Surfactant - Cleansing agent. Cosmetic products used to cleanse the skin utilise the surface-active action that produces a lowering of the surface tension of the stratum corneum, facilitating the removal of dirt and impurities.
Surfactant - Emulsifying agent. Emulsions are thermodynamically unstable and are used to soothe or soften the skin and emulsify, so they need a specific, stabilising ingredient. This ingredient forms a film, lowers the surface tension and makes two immiscible liquids miscible. A very important factor affecting the stability of the emulsion is the amount of the emulsifying agent. Emulsifiers have the property of reducing the oil/water or water/oil interfacial tension, improving the stability of the emulsion and also directly influencing the stability, sensory properties and surface tension of sunscreens by modulating the filmometric performance.
Food
Ingredient included in the list of European food additives such as E433 with emulsifier function and with name
How it is composed
The fatty acid (FA) composition of polysorbate 80 (PS80), a sorbitan oleic acid ester copolymerized with about 20mole of ethylene oxide, is typically characterized by gas chromatography. Here, an alternative method was developed. After saponification with potassium hydroxide the FA fraction was collected with liquid-liquid extraction using methyl-tert-butyl ether. HPLC in combination with a Corona® charged aerosol detector (CAD) was applied for the separation and detection. The method was fully validated in terms of specificity, repeatability, limits of quantification, linearity, range, accuracy and robustness. The characterization of 16 different PS80 batches demonstrated variability regarding their FA composition, with e.g. the amount of oleic acid ranging from 67.8±0.7% to 96.6±1.4%. Furthermore, this study identified petroselinic acid, a double-bond positional isomer to oleic acid in all batches, an FA not known to pharmacopoeias at present. In addition, 11-hydroxy-9-octadecenoic acid, an oxidation product of oleic acid was identified. Structure elucidation was performed by means of HPLC-MS/MS. In addition, the method was expanded to the evaluation of the free FAs. Having determined the entire FA composition, the acid value according to EP and USP can be calculated (2)
Medical - Studies
Chemotherapy-induced nausea and vomiting (CINV) may occur during the acute (0-24 h) or delayed (25-120 h) phase following chemotherapy administration. The addition of a neurokinin 1 receptor antagonist to antiemetic regimens containing a 5-hydroxytryptamine type 3 receptor antagonist and dexamethasone has resulted in improved CINV prophylaxis. Due to numerous adverse events and hypersensitivity reactions associated with fosaprepitant, a commonly used neurokinin 1 receptor antagonist, there remains an unmet need for better-tolerated formulations. HTX-019, the US FDA-approved polysorbate 80- and synthetic surfactant-free aprepitant injectable emulsion, is bioequivalent to and better tolerated (fewer treatment-emergent adverse events) than fosaprepitant. HTX-019 represents a valuable alternative to fosaprepitant for CINV prophylaxis (3).

The presence of blood‐brain barrier (BBB) greatly limits the availability of drugs and their efficacy against glioma. Focused ultrasound (FUS) can induce transient and local BBB opening for enhanced drug delivery. Here, we developed polysorbate 80‐modified paclitaxel‐loaded PLGA nanoparticles (PS‐80‐PTX‐NPs, PPNP) and examined the enhanced local delivery into the brain for glioma treatment by combining with FUS. Our result showed PPNP had good stability, fast drug release rate and significant toxicity to glioma cells. Combined with FUS, PPNP showed a stronger BBB permeation efficiency both in the in vitro and in vivo BBB models. Mechanism studies revealed the disrupted tight junction, reduced P‐glycoprotein expression and ApoE‐dependent PS‐80 permeation collectively contribute to the enhanced drug delivery, resulting in significantly stronger antitumour efficacy and longer survival time in the tumour‐bearing mice. Our study provided a new strategy to efficiently and locally deliver drugs into the brain to treat glioma (4).
Escherichia coli biotype O104:H4 recently caused the deadliest E. coli outbreak ever reported. Based on prior results, it was hypothesized that compounds inhibiting biofilm formation by O104:H4 would reduce its pathogenesis. The nonionic surfactants polysorbate 80 (PS80) and polysorbate 20 (PS20) were found to reduce biofilms by ≥ 90% at submicromolar concentrations and elicited nearly complete dispersal of preformed biofilms. PS80 did not significantly impact in vivo colonization in a mouse infection model; however, mice treated with PS80 exhibited almost no intestinal inflammation or tissue damage while untreated mice exhibited robust pathology. As PS20 and PS80 are classified as 'Generally Recognized as Safe' (GRAS) compounds by the Food and Drug Administration (FDA), these compounds have clinical potential to treat future O104:H4 outbreaks (5).

Stenotrophomonas maltophilia is an opportunistic multiple-drug-resistant human pathogen that forms biofilms on implanted medical devices. We examined the potential inhibitory activity of polysorbate 80 and polymyxin B against S. maltophilia. A combination of subMIC polymyxin B and polysorbate 80 was the most effective inhibitor of growth and biofilm formation (6).
In ophthalmology it is an excipient used as a lubricant in eye drops and eye pads with a mild cleansing action.
Safety
Polysorbate 80 has been involved in isolated cases of allergy in the form of contact dermatitis caused by topical drugs and in other cases after parenteral administration causing generalized reactions such as urticaria-angioedema and anaphylaxis (7).
The Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO)Expert Committee on Food Additives (JECFA)derived an Acceptable Daily Intake (ADI) of 25mg/kg body weight (bw)/day (group ADI for polysorbates 20, 40, 60, 65 and 80) and the Scientific Committee on Food (SCF) derived a group ADI of 10mg/kg bw/day. Small amounts of polyoxyethylene sorbitansare absorbed. Similar toxicokinetics would be expected for all polysorbates based on their similarities in structure and metabolic fate. The acute toxicity is very low. There is no concern regarding genotoxicity, carcinogenicity or developmental toxicity. From a limited number of studies,there is no indication of reproductive toxicity. The Panel recommended that the maximum limits for the impurities of toxic elements (arsenic, lead, cadmium and mercury) in the EC specification for polysorbates (E432–E436) should be revised in order to ascertain that polysorbates (E432–E436) as food additives will not be a significant source of exposure to those toxic elements in food (8).
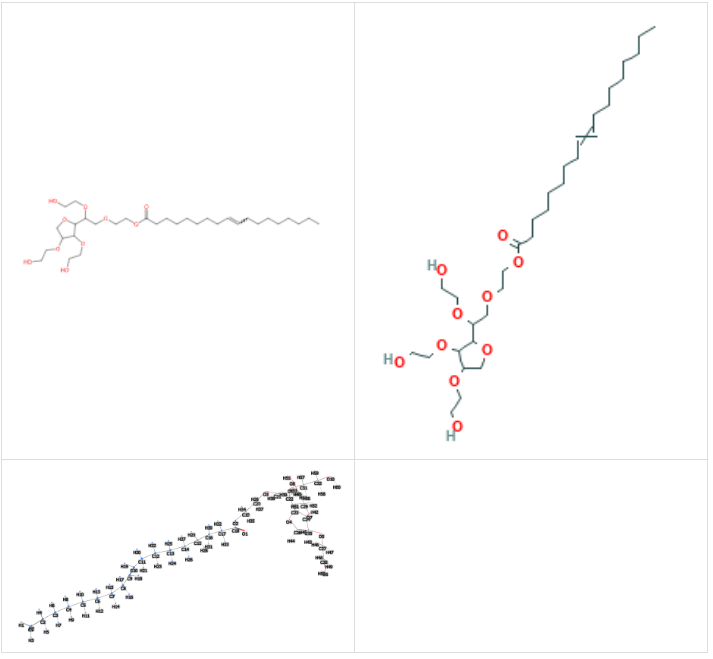
- Molecular Formula C32H60O10
- Molecular Weight 604.8 g/mol
- CAS 9005-65-6
- UNII 6OZP39ZG8H
- EC Number 500-019-9
Synonyms :
Tween(R) 80, Polyoxyethylene 20 sorbitan monooleate, 2-hydroxyethyl 2-deoxy-3,5-bis-O-(2-hydroxyethyl)-6-O-{2-[(9E)-octadec-9-enoyloxy]ethyl}hexofuranoside, sorbate80, Polyoxyethylene 20 sorbitan monooleate, EG Coolant, Sorbitan, mono-9-octadecenoate, poly(oxy-1,2-ethanediyl) derivs, 2-hydroxyethyl 2-deoxy-3,5-bis-O-(2-hydroxyethyl)-6-O-[2-(oleoyloxy)ethyl]hexofuranoside, 2-[2-[3,5-bis(2-hydroxyethoxy)oxolan-2-yl]-2-(2-hydroxyethoxy)ethoxy]ethyl
References______________________________________________________
(1) Narang AS, Delmarre D, Gao D. Stable drug encapsulation in micelles and microemulsions. Int J Pharm. 2007 Dec 10;345(1-2):9-25. doi: 10.1016/j.ijpharm.2007.08.057.
Abstract. Oral absorption of hydrophobic drugs can be significantly improved using lipid-based non-particulate drug delivery systems, which avoid the dissolution step. Micellar and microemulsion systems, being the most dispersed of all, appear the most promising. While these systems show high drug entrapment and release under sink conditions, the improvement in oral drug bioavailability is often unpredictable. The formulation and drug-related biopharmaceutical aspects of these systems that govern oral absorption have been widely studied. Among these, preventing drug precipitation upon aqueous dilution could play a predominant role in many cases. Predictive ability and quick methods for assessment of such problems could be very useful to the formulators in selecting lead formulations. This review will attempt to summarize the research work that could be useful in developing these tools.
Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J.H Strategies to address low drug solubility in discovery and development. . Pharmacol. Rev. 2013, 65, 315–499
(2) Ilko D, Braun A, Germershaus O, Meinel L, Holzgrabe U. Fatty acid composition analysis in polysorbate 80 with high performance liquid chromatography coupled to charged aerosol detection. Eur J Pharm Biopharm. 2015 Aug;94:569-74. doi: 10.1016/j.ejpb.2014.11.018.
(3) Navari RM. HTX-019: polysorbate 80- and synthetic surfactant-free neurokinin 1 receptor antagonist for chemotherapy-induced nausea and vomiting prophylaxis. Future Oncol. 2019 Jan;15(3):241-255. doi: 10.2217/fon-2018-0577.
(4) Li Y, Wu M, Zhang N, Tang C, Jiang P, Liu X, Yan F, Zheng H. Mechanisms of enhanced antiglioma efficacy of polysorbate 80-modified paclitaxel-loaded PLGA nanoparticles by focused ultrasound. J Cell Mol Med. 2018 Sep;22(9):4171-4182. doi: 10.1111/jcmm.13695.
(5) Sloup RE, Cieza RJ, Needle DB, Abramovitch RB, Torres AG, Waters CM. Polysorbates prevent biofilm formation and pathogenesis of Escherichia coli O104:H4. Biofouling. 2016 Oct;32(9):1131-1140. doi: 10.1080/08927014.2016.1230849.
(6) Malinowski AM, McClarty BM, Robinson C, Spear W, Sanchez M, Sparkes TC, Brooke JS. Polysorbate 80 and polymyxin B inhibit Stenotrophomonas maltophilia biofilm. Diagn Microbiol Infect Dis. 2017 Feb;87(2):154-156. doi: 10.1016/j.diagmicrobio.2016.11.008.
(7) Palacios Castaño MI, Venturini Díaz M, Lobera Labairu T, González Mahave I, Del Pozo Gil MD, Blasco Sarramián A. Anaphylaxis Due to the Excipient Polysorbate 80. J Investig Allergol Clin Immunol. 2016;26(6):394-396. doi: 10.18176/jiaci.0109.
(8) EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), 2015. Scientific Opinion on the re‐evaluation of polyoxyethylene sorbitan monolaurate (E 432), polyoxyethylene sorbitan monooleate (E 433), polyoxyethylene sorbitan monopalmitate (E 434), polyoxyethylene sorbitan monostearate (E 435) and polyoxyethylene sorbitan tristearate (E 436) as food additives. Efsa Journal, 13(7), p.4152.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (1)
Component type: Chemical Main substances: Last update: 2023-07-08 18:16:04 | Chemical Risk: |


