L-carnitine, chemically known as 3-hydroxy-4-(trimethylammonium)butanoate, is a quaternary ammonium compound involved in the metabolism of most mammals, plants and some bacteria.
The name defines the structure of the molecule:
- L- refers to the configuration of the carnitine molecule. In chemistry, the terms 'L-' and 'D-' are used to indicate the configuration of a molecule around a specific chiral centre. In the case of carnitine, the 'L-' form is the one that is biologically active and used in supplements and food additives.
- Carnitine is derived from the Latin 'carnus' or meat, as the compound was first isolated from meat.
In the human body, L-carnitine is synthesised from the amino acids lysine and methionine.
The synthesis process takes place in different steps:
- Preparation of epichlorohydrin. Epichlorohydrin is prepared from glycerol, a simple polyol compound, through a series of reactions involving hydrochloric acid and chlorine.
- Reaction with trimethylamine and sodium cyanide. Epichlorohydrin is reacted with trimethylamine and sodium cyanide to form an intermediate compound.
- Hydrolysis and decarboxylation. The intermediate compound undergoes hydrolysis and decarboxylation, a process involving the addition of water and the removal of a carboxyl group, to form L-carnitine.
It appears in the form of a white powder.

What it is used for and where
It is often used as a dietary supplement for its potential benefits in weight loss, brain function, heart health and exercise performance.
Medical
Carnitine is synthesised in many eukaryotic organisms and its biosynthesis is initiated by the methylation of lysine. It plays a key role in the metabolism of fatty acids in the mitochondria (1).
Carnitine, however, is not considered an essential nutrient and is present in many foods, mainly those from animal sources. Lysine and methionine are necessary ingredients for carnitine biosynthesis.
All body tissues can produce deoxy-carnitine but, in humans, the enzyme that enables the hydroxylation of deoxy-carnitine to carnitine is only found in the liver, brain and kidneys (2).
Cosmetics
It performs a number of interesting acts.
Anti-static agent. Static electricity build-up has a direct influence on products and causes electrostatic adsorption. The antistatic ingredient reduces static build-up and surface resistivity on the surface of the skin and hair.
Hair conditioning agent. A significant number of ingredients with specific and targeted purposes can co-exist in a hair shampoo: cleansers, conditioners, thickeners, mattifying agents, sequestering agents, fragrances, preservatives, special additives. However, the indispensable ingredients are the cleansers and conditioners as they are necessary and sufficient for hair cleansing and manageability. The others have commercial and non-essential accessory acts such as: appearance, perfume, colouring, etc. Hair conditioning agents have the task of increasing shine, manageability and volume, and reducing static electricity, especially after treatments such as colouring, ironing, waving, drying and brushing. They are, in practice, dispersants that may contain cationic surfactants, thickeners, emollients, polymers. The typology of hair conditioning agents includes: intensive conditioners, instant conditioners, thickening conditioners, drying conditioners.
Surfactant - Cleansing agent. Cosmetic products used to cleanse the skin utilise the surfactant action that produces a lowering of the surface tension of the stratum corneum, facilitating the removal of dirt and impurities.
Surfactant - Foam booster. This has the function of introducing gas bubbles into the water for a purely aesthetic factor, which does not affect the cleansing process, but only satisfies the commercial aspect of the cleanser by helping to spread the cleanser on the hair. This helps in the commercial success of a shampoo formulation. Since sebum has an inhibiting action on the bubble, more foam is produced in the event of a second shampoo.
Viscosity-increasing agent, aqueous. Since viscosity is important to increase the chemical and physical stability of the product, Viscosity Enhancing Agent, aqueous is an important dosage factor in gels, suspensions, emulsions, solutions. Increasing viscosity makes formulations less sedimentary and more homogeneously thickened.
The most relevant studies on carnitine have been selected with a summary of their contents:
Carnitine studies

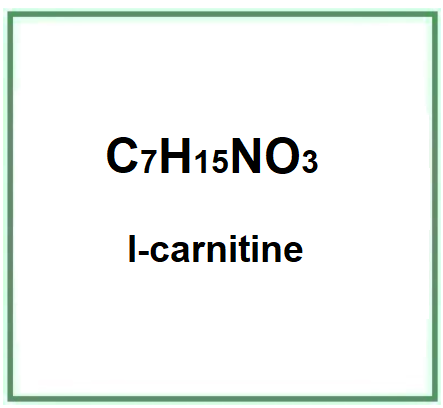
- Molecular Formula C7H15NO3
- Molecular Weight 161.20 g/mol
- CAS 541-15-1
- UNII 0G389FZZ9M
- EC Number 208-768-0
References____________________________________________________________________
(1) Bremer J. Carnitine—metabolism and functions. Physiol. Rev. 1983;63:1420–80.
(2) Jacob C, Belleville F. L-carnitine: metabolism, functions and value in pathology. Pathol Biol (Paris). 1992 Nov;40(9):910-9.
![]() L-carnitine
L-carnitine