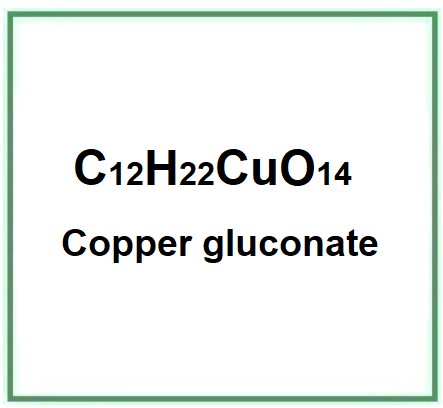
![]() Rame gluconato
Rame gluconato
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
10 pts from Al222
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about Rame gluconato Review Consensus 10 by Al222 (20707 pt) | 2023-Jul-14 14:11 |
| Read the full Tiiip | (Send your comment) |
Copper gluconate is a chemical compound, a copper salt of D-gluconic acid.
The name "copper gluconate" is derived from its chemical composition: a copper ion (Cu2+) combined with a gluconate ion (C6H11O7-).
Copper gluconate is typically synthesized by reacting copper carbonate or copper hydroxide with gluconic acid. The reaction is performed in an aqueous solution, and the copper gluconate precipitates out of the solution. The precipitate is then collected, washed, and dried to obtain the pure compound.
Here is a simple example of how this can be done:
- Preparation of solutions. A solution of copper carbonate or copper hydroxide and a solution of gluconic acid.
- Reaction. By adding the gluconic acid solution to the copper carbonate or copper hydroxide solution, the reaction produces copper gluconate and water
- Isolation of product: The copper gluconate precipitate can be collected by filtration, then washed and dried to obtain the pure compound
It appears as a blue or light blue powder or liquid.

What it is for and where
Copper gluconate is often used as a copper supplement. Copper is an essential trace element that is vital to the health of all living things (humans, plants, animals, and microorganisms).
Cosmetics
- Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
- Skin protectant. It creates a protective barrier on the skin to defend it from harmful substances, irritants, allergens, pathogens that can cause various inflammatory conditions. These products can also improve the natural skin barrier and in most cases more than one is needed to achieve an effective result.
Dietary Supplement
Copper gluconate is used as a dietary supplement to prevent or treat copper deficiency. Copper is an essential trace mineral necessary for survival. It is found in all body tissues and plays a role in making red blood cells and maintaining nerve cells and the immune system.
Food Additive
It's used as a food additive to enrich foods with copper.
Pharmaceuticals
In the pharmaceutical industry, copper gluconate is used in products designed to prevent or treat copper deficiencies.
Animal Feed
It's also used as a supplement in animal feed to ensure animals get adequate copper in their diet.

- Molecular Formula C12H22CuO14
- Molecular Weight 453.84 g/mol
- CAS 527-09-3
- UNII RV823G6G67
- EC Number 208-408-2 235-844-0
- Nikkaji J6.667B
Compendium of the most significant studies with reference to properties, intake, effects.
García-Martínez BA, Montes S, Tristán-López L, Quintanar-Guerrero D, Melgoza LM, Baron-Flores V, Ríos C. Copper biodistribution after acute systemic administration of copper gluconate to rats. Biometals. 2021 Jun;34(3):687-700. doi: 10.1007/s10534-021-00304-1.
Abstract. Neurodegenerative disorders have been linked to the decrease of copper concentrations in different regions of the brain. Therefore, intake of micronutrient supplements could be a therapeutic alternative. Since the copper distribution profile has not been elucidated yet, the aim of this study was to characterize and to analyze the concentration profile of a single administration of copper gluconate to rats by two routes of administration. Male Wistar rats were divided into three groups. The control group received vehicle (n = 5), and the experimental groups received 79.5 mg/kg of copper orally (n = 4-6) or 0.64 mg/kg of copper intravenously. (n = 3-4). Blood, striatum, midbrain and liver samples were collected at different times. Copper concentrations were assessed using atomic absorption spectrophotometry. Copper concentration in samples from the control group were considered as baseline. The highest copper concentration in plasma was observed at 1.5 h after oral administration, while copper was quickly compartmentalized within the first hour after intravenous administration. The striatum evidenced a maximum metal concentration at 0.25 h for both routes of administration, however, the midbrain did not show any change. The highest concentration of the metal was held by the liver. The use of copper salts as replacement therapy should consider its rapid and discrete accumulation into the brain and the rapid and massive distribution of the metal into the liver for both oral and intravenous routes. Development of controlled-release pharmaceutical formulations may overcome the problems that the liver accumulation may imply, particularly, for hepatic copper toxicity.
Abe M, Usuda K, Hayashi S, Ogawa I, Furukawa S, Igarashi M, Nakae D. Carcinogenic risk of copper gluconate evaluated by a rat medium-term liver carcinogenicity bioassay protocol. Arch Toxicol. 2008 Aug;82(8):563-71. doi: 10.1007/s00204-008-0294-x.
Abstract. Carcinogenic risk and molecular mechanisms underlying the liver tumor-promoting activity of copper gluconate, an additive of functional foods, were investigated using a rat medium-term liver carcinogenicity bioassay protocol (Ito test) and a 2-week short-term administration experiment. In the medium-term liver bioassay, Fischer 344 male rats were given a single i.p. injection of N-nitrosodiethylamine at a dose of 200 mg/kg b.w. as a carcinogenic initiator. Starting 2 weeks thereafter, rats received 0, 10, 300 or 6,000 ppm of copper gluconate in diet for 6 weeks. All rats underwent 2/3 partial hepatectomy at the end of week 3, and all surviving rats were killed at the end of week 8. In the short-term experiment, rats were given 0, 10, 300 or 6,000 ppm of copper gluconate for 2 weeks. Numbers of glutathione S-transferase placental form (GST-P) positive lesions, single GST-P-positive hepatocytes and 8-oxoguanine-positive hepatocytes, and levels of cell proliferation and apoptosis in the liver were significantly increased by 6,000 ppm of copper gluconate in the medium-term liver bioassay. Furthermore, hepatic mRNA expression of genes relating to the metal metabolism, inflammation and apoptosis were elevated by 6,000 ppm of copper gluconate both in the medium-term liver bioassay and the short-term experiments. These results indicate that copper gluconate possesses carcinogenic risk toward the liver at the high dose level, and that oxidative stress and inflammatory and pro-apoptotic signaling statuses may participate in its underlying mechanisms.
Cai DH, Zhang CL, Liu QY, He L, Liu YJ, Xiong YH, Le XY. Synthesis, DNA binding, antibacterial and anticancer properties of two novel water-soluble copper(II) complexes containing gluconate. Eur J Med Chem. 2021 Mar 5;213:113182. doi: 10.1016/j.ejmech.2021.113182.
Abstract. In this paper, two new Cu(II) complexes, [Cu(Gluc)(HPB)(H2O)]Gluc (CuG1) and [Cu(Gluc)(HPBC)(H2O)]Gluc (CuG2) (where HPB = 2-(2'-pyridyl)benzimidazole, HPBC = 5-chloro-2-(2'-pyridyl)benzimidazole, Gluc = d-Gluconic acid), with good water solubility were synthesized and characterized. These complexes exhibited a five-coordinated tetragonal pyramidal geometry. The DNA binding and cleavage properties of the complexes were investigated using multi-spectroscopy, viscosity measurement, molecular docking and gel electrophoresis analysis methods. The results showed that the complexes could interact with DNA by insertion and groove binding, and cleave CT-DNA through a singlet oxygen-dependent pathway in the presence of ascorbic acid. The studies on antibacterial and anticancer activities in vitro demonstrated that both complexes had good inhibitory activity against three Gram-positive bacteria (Staphylococcus aureus, Bacillus subtilis, Listeria monocytogenes) and one Gram-negative bacterium (Escherichia coli) and good cytotoxic activity toward the tested cancer cells (A549, HeLa and SGC-7901). CuG2 showed higher antimicrobial and cytotoxic activities than CuG1, which was consistent with their binding strength and cleavage ability to DNA, indicating that their antimicrobial and cytotoxic activities may be related to the DNA interaction. Moreover, the cell-based mechanism studies have indicated that CuG1 and CuG2 could arrest the cell cycle at G2/M phase, elevate the levels of intracellular reactive oxygen species (ROS) and decrease the mitochondrial membrane potential (MMP). The results showed that the complexes could induce apoptosis through DNA-damaged and ROS-mediated mitochondrial dysfunction pathways. Finally, the in vivo antitumor study revealed that CuG2 inhibited tumor growth by 50.44%, which is better than that of cisplatin (40.94%). Copyright © 2021 Elsevier Masson SAS.
Curcio, A., Romano, A., Marchitto, N., Pironti, M., & Raimondi, G. (2018). Efficacy and Safety of a New Formulation of Ferric Sodium EDTA Associated with Vitamin C, Folic Acid, Copper Gluconate, Zinc Gluconate and Selenomethionine Administration in Patients with Secondary Anaemia. J Blood Lymph, 8(224), 2.
Abstract. Anemia is a global problem since two billion people are affected by blood cells disorders. Anemia may reduce the quality of life of affected patients and may also to get worse the outcome and quality of life of patients with comorbidities as kidney failure, heart failure, arrhythmia, coronary heart disease and so on. In patients with coronary heart disease, anginal episodes may increase in frequency and severity, and patients with kidney failure may have an increased number of re-hospitalizations. Here we report the effectiveness of the therapy with Ferric Sodium EDTA, in combination with vitamin C, folic acid, copper gluconate, zinc gluconate and selenomethionine (FERACHEL FORTE®) that has shown several advantages in daily clinical practice.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (1)
Component type: Chemical Main substances: Last update: 2023-07-14 13:30:59 | Chemical Risk: |